
The GPx activity of human selenoproteins, SELENOM and SELENOW
Glutathione Peroxidases (GPXs) are a family of selenium-containing antioxidant enzymes that catalyze the reduction of a variety of hydroperoxides (ROOH) in the presence of reduced glutathione (Scheme 1)
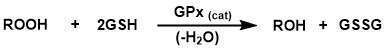
Scheme 1. GPx catalyzed reduction of hydroperoxides
The human body contains twenty five selenoproteins,1 yet the biological function of many of these proteins remains unclear or poorly studied. For example, human selenoprotein M (SELENOM) and W (SELENOW) are still biologically uncharacterized and their functions are not established. SELENOM is an endoplasmic reticulum (ER) selenoprotein which is most abundant in the brain,2 suggesting an important role in the nervous system, while SELENOW, is a cytosolic protein that is highly conserved in mammals, and is one of the most highly expressed selenoproteins.3 In order to understand the function of SELENOM and SELENOW, previously our group have presented the total chemical synthesis of these two selenoproteins by utilizing SPPS and native chemical ligations.4
Furthermore, herein, we are presenting the glutathione peroxidase activities and redox potential of these two selenoproteins. The antioxidant activities have been performed spectrophotometrically using coupled reductase assay. Our preliminary data for the glutathione peroxidase (GPx) activities of SELENOM and SELENOW, were compared to natural bovine GPx enzyme and synthetic GPx mimic.
[1] G. V. Kryukov, S. Castellano, S. V. Novoselov, A. V. Lobanov, O. Zehtab, R. Guigo, V. N. Gladyshev, Science. 2003, 300, 1439–1443.
[2] K. V. Korotkov, S. V. Novoselov, D. L. Hatfield, V. N. Gladyshev, Mol. Cell. Biol. 2002, 22, 1402–1411.
[3] J. Y. Yeh, M. A. Beilstein, J. S. Andrews, P. D. Whanger, FASEB J. 1995, 9, 392–396.
[4] L. Dery, P. S. Reddy, S. Dery, R. Mousa, O. Ktorza, A. Talhami, N. Metanis, Chem. Sci. 2017, 8, 1922–1926.
Powered by Eventact EMS