
Total synthesis of novel pleuromutilin analogs
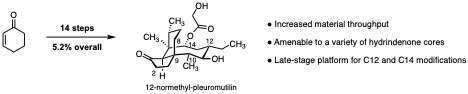
(+)-Pleuromutilin is a diterpene fungal metabolite that exhibits antibacterial activity against Gram-positive bacteria. Pleuromutilin antibiotics target the bacterial ribosome and are associated with low rates of resistance development. Owing to the difficulties in accessing fully-synthetic pleuromutilin analogs, a majority of the pleuromutilins synthesized to date differ only in their substitution at C22. Recently, derivatives of pleuromutilin bearing an epimerized C12 stereocenter have exhibited extended spectrum antibiotic activity. Over the past fifty years, extensive semi-synthetic efforts have led to four clinically-relevant pleuromutilin analogs differing only at the glycolic acid side chain. Additionally, pleuromutilins are rapidly oxidized in vivo by cytochrome P450 at C2 and C8. These oxidized products lack antibiotic activity and are rapidly cleared from the body. In an effort to more efficiently access extended-spectrum and metabolically-stable pleuromutilin analogs, we have developed a novel total synthesis of (+)-pleuromutilin that is amenable to both early- and late-stage modifications. This route utilizes a [2,3]-rearrangement to set the challenging C9 stereocenter, followed by reductive cyclization of an elaborated ynal to form the 8-membered macrocycle. This route is tolerant to a variety of modifications on the hydrindane core and affords a platform for late-stage diversification at both C14 and C12.
Powered by Eventact EMS