
Aqueous macrocyclic N4O4 chelates of tetravalent uranium
2Nuclear Energy Division, Radiochemistry & Processes Department, The French Alternative Energies and Atomic Energy Commission (CEA), Bagnols Sur Cèze, France
3Chemistry Department, Nuclear Research Centre Negev, Beer-Sheva, Israel
4Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
5Chemical Engineering Departmnet, Ben-Gurion University of the Negev, Beer-Sheva, Israel
The coordination chemistry of lanthanides and early actinides complexes in aqueous solutions is of great interest both for understanding basic chemistry as well for applications in environmental and/or medical hazards-decontamination.
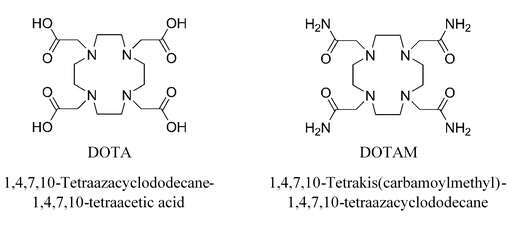
We have recently reported of our study regarding the mono-capped square antiprismatic [UIVDOTA(Lax)] complex and the effect of the axial ligand (Lax) substitution on the complex electronic configuration and redox properties in aqueous solutions1. The UIV cation coordinative sphere in this complex is saturated by the DOTA4- chelate, through its four N-donor and four carboxylate groups (N4O4), and an axially bonded small ligand such as H2O, OH- and F-. The rigid macrocyclic coordination of the UIV cation inhibits its thermodynamically-favored oxidation by dissolved dioxygen in aqueous solutions. We look to further investigate the use of likewise N4O4-framework macrocyclic ligands as potential chelates for the UIV cation. Such compounds are mostly discussed today with regard to their metaloorganic characteristics in non-aqueous solutions and were shown before to introduce some exceptional properties to the chelated UIV and UV cations2. Elaborate study of similar complexes in aqueous solutions may hold great prospect for development of novel nuclear fuel cycle related processes such as nuclear waste treatment techniques.
We herein report of both the deciphered crystal structure of the [UIVDOTA(F)]- complex and the synthesis and characterization (UV-VIS, 1H-NMR, electrochemistry) of the [UIVDOTAM]4+ complex, another N4O4-framwork macrocyclic complex. The substitution of the UIV coordination by carboxylate groups of the DOTA4- chelate in favor of amide O-donor groups of the DOTAM chelate has great effect on the cation stability in aqueous solutions.
- Dovrat et al., Chemistry an European J. submitted
- Hümmer et al., Chem. 2017, 56, 6, 3201-3206
Powered by Eventact EMS