
One-pot stereoselective synthesis and mechanism of formation of nucleosides-2’,3’-Cyclic Thio/Seleno-Phosphate
1
2
1
1Department of Chemistry, Bar-Ilan University, Ramat-Gan, Israel
2Pharmacenter Bonn, Pharmaceutical Institute, University of Bonn, Bonn, Central District, Germany
2Pharmacenter Bonn, Pharmaceutical Institute, University of Bonn, Bonn, Central District, Germany
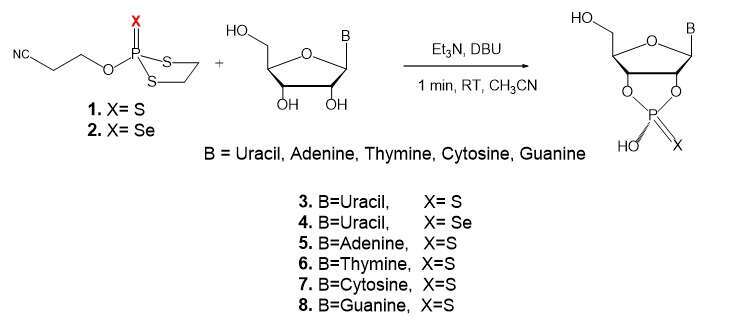
A new facile, rapid, stereo- and regio-selective one-pot synthesis of nucleoside-2’,3’-O,O-phosphorothioate/selenoate has been developed. The method is based on the DBU-assisted ring-opening of a new reagent, 2-cyanoethoxy-thio/seleno–dithiophospholane, by uridine producing in a good yield Sp-uridine 2’,3’-O,O-phosphorothioate/selenoate with no need of chiral reagents or chiral separations. This synthetic method was applied also to all four natural nucleosides. We have deciphered the origin of stereo- and regio-selectivity of the reaction by monitoring it with 31P NMR. The kinetic product is the Sp isomer, while at elevated temperatures both Sp and Rp isomers are obtained.
Powered by Eventact EMS