
Informing titanium dioxide trapped states: stoichiometric studies on kinetic reactivity and proton coupled electron equilibrium
2Department of Chemistry and Department of Molecular Biology and Biochemistry, University of California, Irvine, California, USA
3Department of Chemistry and Chemical Biology and National Biomedical Center for Advanced ESR Technology, Cornell University, Ithaca, New York, USA
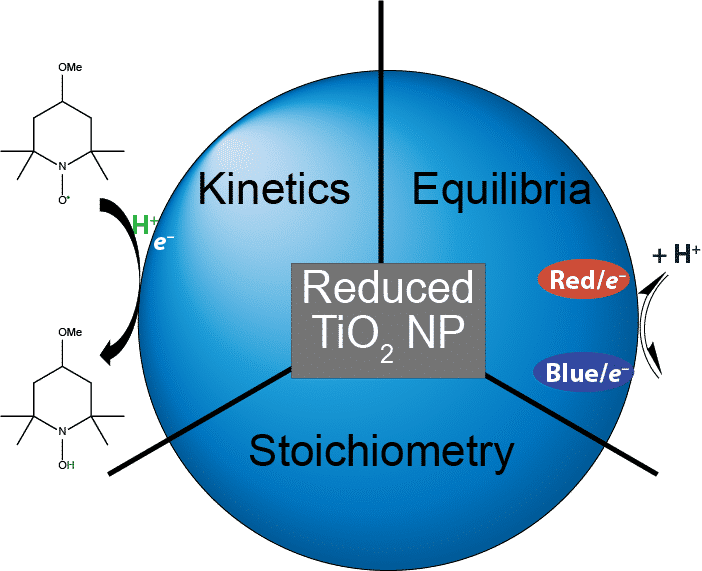
Titanium dioxide (TiO2) is a widely used photocatalyst and redox mediator due to its earth abundance, chemical stability, and ability to hold and transport multiple redox equivalents. Nanoparticles (NPs) of TiO2 have a high surface to volume ratio, making interfacial interactions, surface reactivity, and electronic properties more pronounced. Upon photoexcitation and rapid hole quenching, electrons on TiO2 NPs can be stabilized in electron traps (trapped states). Such trapped states have been widely invoked but are little understood. Traditional approaches have described processes involving electrons as electron transfer (ET) only, however more recent reports have shown that much of the redox chemistry of TiO2 actually proceeds via proton-coupled electron transfer (PCET).1 In the following studies, aqueous TiO2 NP colloids were photochemically reduced in the presence of hole-quenching alcohols, resulting in trapped electrons that are stable for days in anaerobic atmosphere. Prior work on these NPs showed two spectroscopically distinct, thermally equilibrated classes of trapped electrons, called Red/e– and Blue/e–.2 In the presented studies, stoichiometric evaluations of both the PCET kinetic reactivity and trapped state equilibria as a function of proton concentration were explored. Reactivity studies showed a preferential reactivity of Red/e– with PCET reagent 4-MeO-TEMPO followed by a slow re-equilibration after the reaction. Proton equilibria studies revealed a trapped state distribution has a stoichiometric dependence on proton or deuteron concentration. Methods of study include UV-visible spectroscopy, stopped flow kinetics, freeze-quench, continuous-wave Electron Paramagnetic Resonance (EPR), and pulsed EPR. These studies are starting to provide fundamental insights into the role of protons in the stoichiometry and reactivity of these trap states.
(1) Science. 2012, 336, 1298. (2) JACS. 2017, 13, 2868.
Powered by Eventact EMS