
Glycosaminoglycans sulfation pattern governs interactions with heavy metal ions
2Medicinal Chemistry, Freie Universität, Berlin, Germany
Glycans are the most abundant biopolymers in nature. They serve for cellular communication, as immune response mediators, have a major structural role and are used for energy storage. Glycans have many applications in medicinal, material science, nanotechnology, and diagnostics. Sulfated saccharides are extracellular components. They interact with many partners in their environment including metal ions. The specific sulfation pattern of a saccharide may play a crucial role in its affinity toward metal ions. Studying the influence of sulfation patterns on metal ion binding stems from: 1) low accessibility to the saccharides and 2) shortage in suitable analytical methods to sense these low concentration interactions. We used electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) to elucidate the selective binding of differently sulfated oligohyaluronans to the heavy metal ions cadmium, lead and mercury. Our results indicate that sulfation pattern controls the preferences of the saccharides toward the different ions. This proved that sulfated saccharides nanolayers may provide selective metal ions sensing and might be used for both environmental and medical related applications. Our work show the potential of using electrochemical tools to study the interactions of saccharides with very low concentrations of ions. The tools we developed can be used to study these very common interactions that are very hard to sense otherwise.
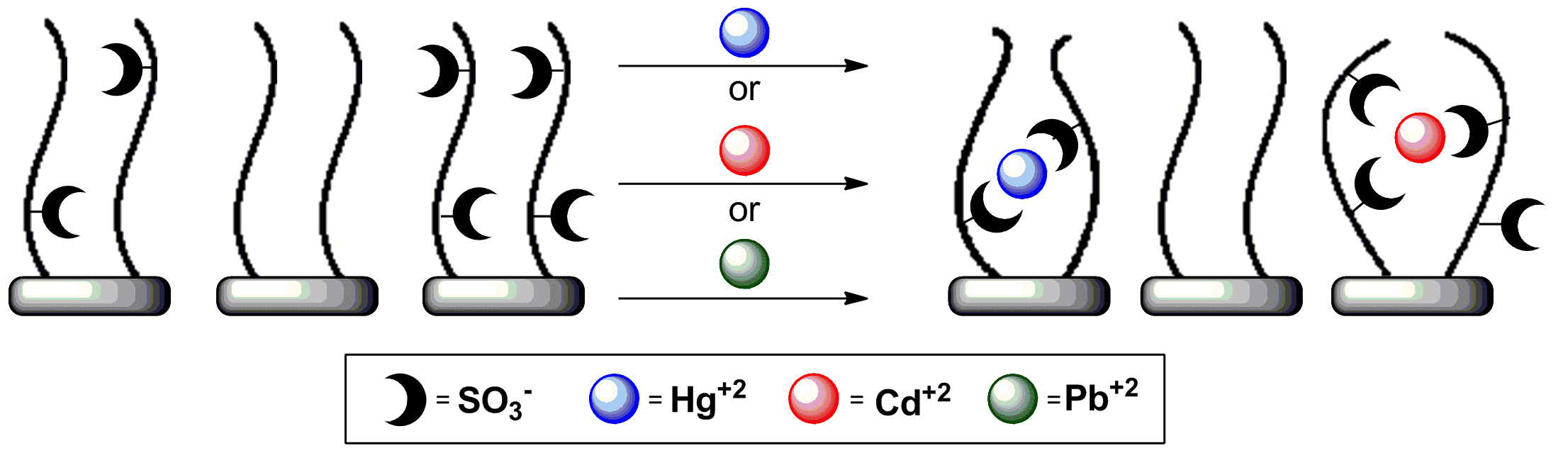
Powered by Eventact EMS