
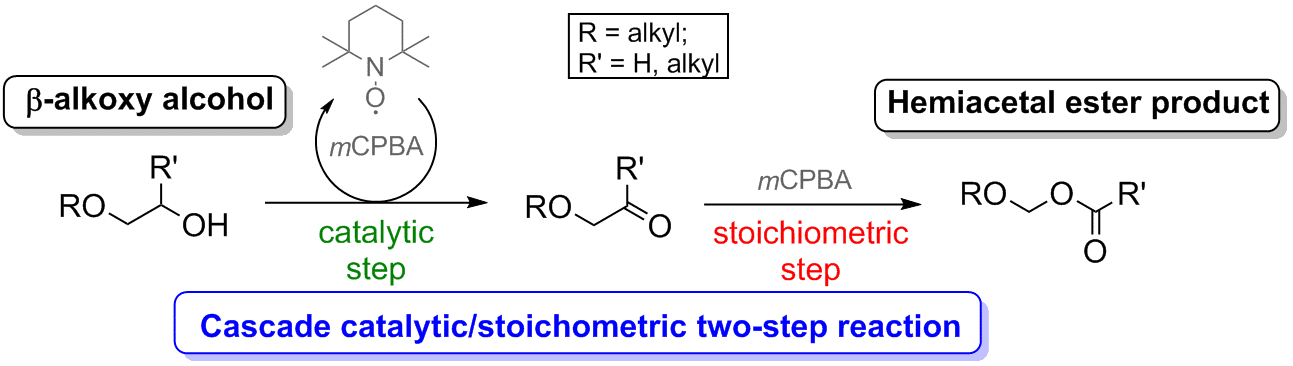
Domino oxidation of β-oxy alcohols, combining organocatalytic and stoichiometric steps.
We established that primary and secondary β-alkoxy alcohols can be cleanly and efficiently oxidized into hemiacetal esters in a cascade two-step process. mCPBA serves both as a stoichiometric oxidant in the first TEMPO-catalyzed step, converting alcohols to aldehydes/ketones, and as a reagent in the second Baeyer-Villiger stoichiometric oxidation, transforming the aldehydes/ketones into hemiacetal esters (Scheme 1). The use of an oxidant common to both steps enables the domino reaction to proceed under a single experimental setting. We also found that longer oxidative cascade sequences are possible when this new methodology is applied to suitable substrates.
In addition, other types of β-oxy alcohols were examined, including (but not limited to) β-acyloxy alcohols, alcohols in which the β-oxygen is part of an acetal system, and alcohols in which two β-oxygens are present, both part of an acetal system. Applying the abovementioned conditions on these substrates gives either carboxylic acid products or decomposition products of 1,1,1-tricarbinol derivatives, but no hemiacetal esters. These results suggest the reaction outcome is highly dependent on the nature of the β-oxygen, namely its electronic density. While electron-rich β-oxy substrates favor the formation of hemiacetal ester products, less electron-donating β-oxy substrates favor formation of carboxylic acids. in other cases, unstable intermediates related to hemiacetal formats are formed, but undergo rapid decomposition.
Powered by Eventact EMS