
Electro-catalytic water oxidation by rigid metal-metal bonded Na3[Ru2(µ-CO3)4]
2Department of Chemistry, Nuclear Research Centre Negev, Beer-Sheva, Israel
3Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
Carbonate in the presence of transition metal cations acts as catalyst/cocatalyst for the oxygen evolution reaction (OER) from water.1 In neutral media (pH 7.0) [RuIIIRuII(µ-CO3)4]3- undergoes one electron oxidation to form [RuIIIRuIII(µ-CO3)4(OH)2]4- at an E1/2 of 0.83 V vs NHE followed by electro-catalytic water oxidation at a potential ≥ 1.5 V. When the same electrochemical measurements are performed in bicarbonate containing media (pH 8.3) the first step remains the same, but two more waves are observed in the cyclic voltammetry before OER. In the 2nd step at 1.18 V the complex undergoes one electron oxidation and ligand exchange (HCO3-) to form [RuIIIRuIV(µ-CO3)4(O)(CO3)]4- which is further oxidized through decomposition to form [RuIVRuIV(µ-CO3)4(O)(O)]4- at 1.35 V which takes part in the process (Fig. 1). The involvement of bicarbonate/carbonate in the OER process is demonstrated by the increase of the catalytic current with the increase in bicarbonate concentration. The mechanism has been established by density functional theory (DFT) calculations.
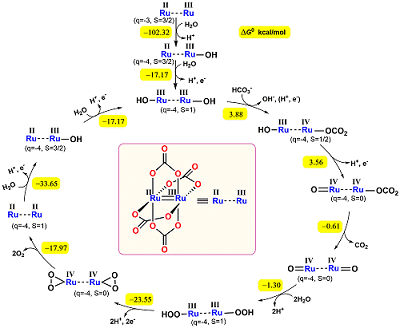
Fig. 1 Mechanism of the electrocatalytic water oxidation by [RuIIIRuII(µ-CO3)4]3-.
Acknowledgements: This research was enabled by a grant from the Pazy Foundation.
References
[1] S. G. Patra, E. Illés, A. Mizrahi and D. Meyerstein, Chem.: Eur. J. 2019, in press.
Powered by Eventact EMS