
Altering the selectivity from bacterial to viral RNA — design, synthesis and evaluation of a novel kanamycin A analogue
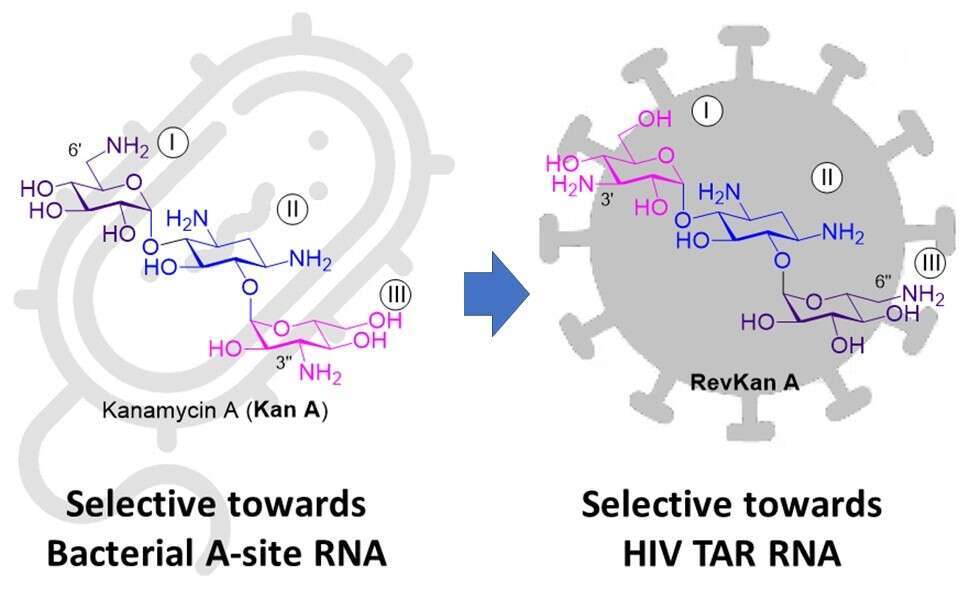
Aminoglycosides (AGs) are a family of pseudo-oligosaccharides that are in clinical use since 1940s as broad-spectrum antibiotics. They exert antibacterial activity by binding to the ribosomal RNA (rRNA) at the decoding A-site, perturbing protein synthesis due to miscoding and translation inhibition, which eventually causes the bacteria death. In addition to the A-site rRNA, AGs also bind to other pathogenic RNAs, e.g. the HIV-1 trans-acting response element (TAR) RNA. During virus replication, HIV TAR RNA will form a transcriptional elongate factor by complexing with trans-activator protein (Tat). The binding of AG to HIV TAR RNA causes a conformational change on the RNA that impairs the elongate factor assembly, hence reducing the frequency of virus DNA transcription.
In general, natural AGs do not have significant selectivity between the bacterial A-site rRNA and the HIV TAR RNA; they usually exhibit strong binding towards both RNAs. The selectivity of AG binding to different RNAs has strong implications to the toxicity of these drugs. In 2014, our lab reported that one of the main factors affecting the toxicity of AGs is their strong binding to the mammalian mitochondrial ribosomes, which are very similar in terms of rRNA A-site structure to the bacterial ribosomes. We therefore anticipated that lowering the affinity to the mitochondrial/bacterial ribosomes should lead to a lower toxicity for the new derivatives. We have designed and synthesized the new semisynthetic AG, RevKan A, which demonstrates very low affinity to the bacterial A-site RNA, while exhibits significantly high affinity to HIV TAR RNA. The observed selectivity of the newly developed RevKanA molecule can employed as an important advantage in anti HIV drug development.
Powered by Eventact EMS