
Macrocyclic oligofurans
Aromatic macrocycles are regarded as models for infinitely conjugated π systems with inner cavities, and exhibit unique optical and magnetic behaviors. This renders them interesting candidates for various future applications in organic and molecular electronics and for the study of host–guest interactions, aggregation, and self-assembly on surfaces.1, 2 While macrocyclic oligothiophenes (nCT) were extensively explored – both computationally and experimentally – macrocyclic oligofurans (nCF) were unknown prior to our work.3, 4
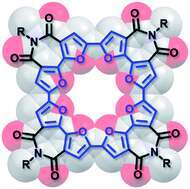
Over the past several years, we have investigated macrocyclic oligofurans. Our initial computational studies highlighted the unique properties of small macrocyclic oligofurans, which are markedly different from those of their thiophene analogs, and aided us to focus our synthetic efforts on potential candidates. Following our computational results, we introduced the first macrocyclic oligofuran, and its X-ray structure revealed a completely planar conformation, as predicted by calculation. The macrocycle formed ordered supramolecular aggregates in solution and in the solid liquid interface.
- J. Krömer, I. Rios-Carreras, G. Fuhrmann, C. Musch, M. Wunderlin, T. Debaerdemaeker, E. Mena-Osteritz and P. Bäuerle, Angew. Chem. Int. Ed., 2000, 39, 3481-3486.
- M. Iyoda and H. Shimizu, Chem. Soc. Rev., 2015, 44, 6411-6424.
- O. Dishi and O. Gidron, J. Org. Chem., 2018, 83, 3119-3125.
- S. V. Mulay, O. Dishi, Y. Fang, M. R. Niazi, L. J. W. Shimon, D. F. Perepichka and O. Gidron, Chem. Sci., 2019, 10, 8527-8532.
Powered by Eventact EMS