
Catalysts for black phosphorous synthesis: ab initio density functional study
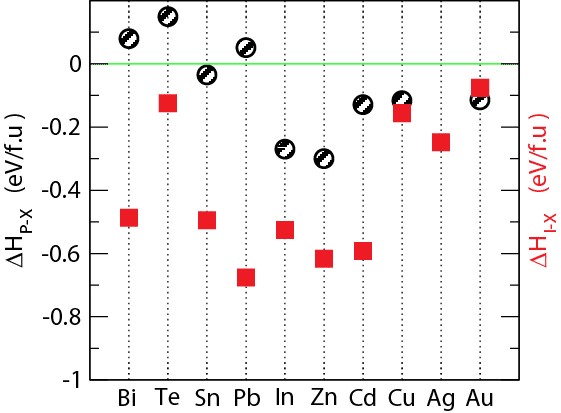
Black phosphorus (BP) has been an emerging layered semiconductor material due its unique structures, higher carrier mobility and optimal band gap for optoelectronic applications. These outstanding properties of BP have been focused on synthesis of large-scale high-quality BP at atmospheric pressure. To date BP is synthesized using CVD and precursors of RP, Sn or AuSn, and SnI4. Other elemental metals along with I2 leads to no BP or below 10% BP growth. Sn is an essential material for the growth of BP, but the Sn-based growth process is expensive due to the slow growth-rate and separation of the by-product from BP. Development of a better catalyst is hindered by insufficient understanding of the BP growth process. Our previous DFT studies reveal that BP nucleus is higher energy due to presence of large number of high-energy strained bonds at the edge compared to RP. This motivates us to investigate the role of the catalyst for BP growth. Our recent studies show 2D materials (graphene and TMDCs) have weak vdW interaction with BP and cannot be used for stabilizing BP. Metals (Bi, Pb, Te) also show weak vdW interaction whereas In, Cd show covalent interaction indicating these are not suitable for BP synthesis. Sn shows the optimal interaction energy for BP stabilization compared to RP, but I2 is still necessary for reducing the edge energy of BP nucleus. Examination of the heat of formation energy (ΔH) of metal phosphides shows that for all elements, ΔH is either positive or smaller than -0.1eV/f.u except for Sn for which ΔH is -0.03 meV/f.u (as shown in figure). Au, Cu, Cd, and Te have lowest ΔH for iodide formation compared to other elements, suggesting that alloying with elements with positive ΔH and elements with ΔH <-0.1eV/f.u can leads to better yields of the BP product.
Powered by Eventact EMS