
Enantioselective synthesis of (α-CF3)diarylmethanes via Ni-catalyzed cross-coupling reactions of organotitanates
Synthetic approaches towards chiral organic compounds bearing trifluoromethyl-substituted stereocenters are of great interest for agrochemical and pharmaceutical labs and industries in their search for new bioactive materials.1 Unsymmetrically substituted 1,1-diarylalakane unit is an important structural motif among numerous drugs and drug candidates.2
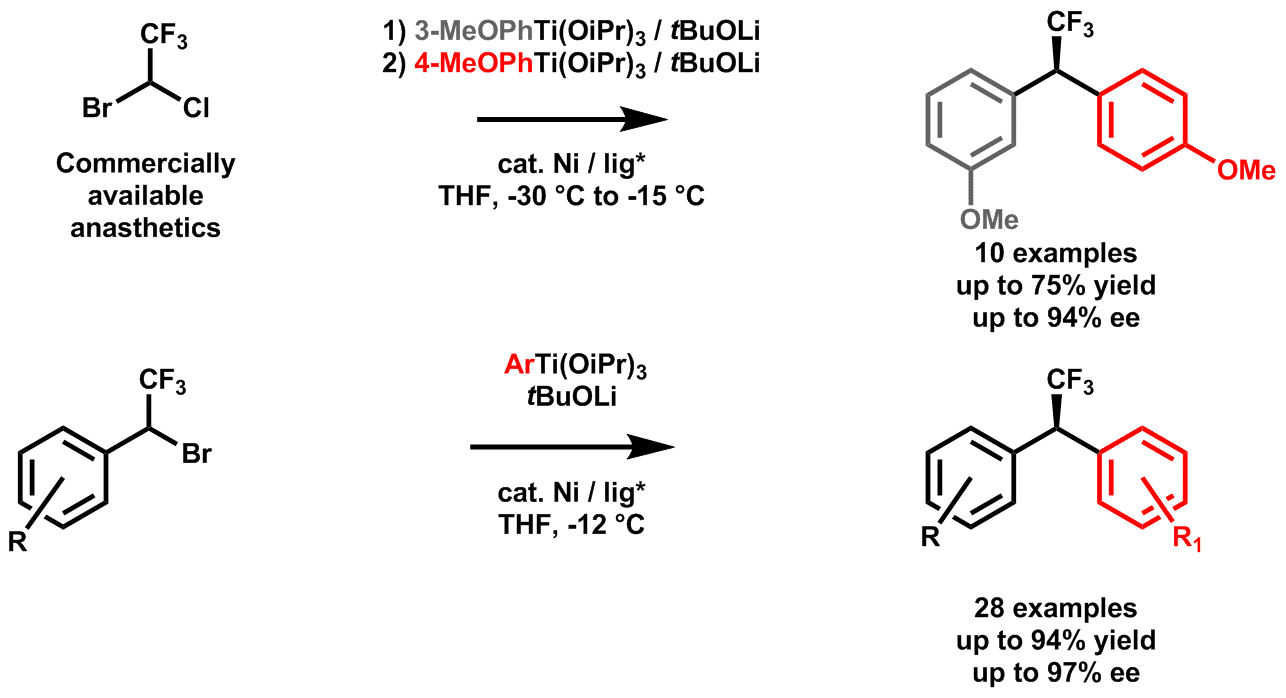
Recently during our work on utilization of bisfunctionalized electrophiles, bearing both a trifluoromethyl and a functional group as direct substituents of the reactive center, in enantioselective cross-coupling reactions3 we discovered superiority of aryltitanium nucleophiles over classical widely employed organozinc and -magesium analogues.4 In this work we report on the catalytic enantioselective synthesis of (α-trifluoromethyl)diarylmethanes enabled by use of organotitanates. While other organometal derivatives nearly fail to provide a desired product or have severe limitations5, utilization of organotitaniums gives access to desired products in excellent yields and enantioselectivities.6
- Y. Zhou, J. Wang, Z. Gu et. al.; Chem. Rev. 2016, 116, 422.
- F. Schmidt, R. T. Stemmler, J. Rudolph, C. Bolm; Chem. Soc. Rev, 2006, 35, 454.
- A. Varenikov, M. Gandelman; Nature Communications, 2018, 9, 3566.
- A. Varenikov, M. Gandelman; J. Am. Chem. Soc., 2019, 141, 10994.
- W. Huang, M. Hu, X. Wan, Q. Shen; Nature Communications, 2019, 10, 2963.
- A. Varenikov, M. Gandelman; Manusctript in preparation.
Powered by Eventact EMS