
Hierarchical assembly pathways of spermine induced tubulin conical-spiral architectures
2The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem, Israel
3The Institute for Drug Research, The Hebrew University of Jerusalem, Jerusalem, Israel
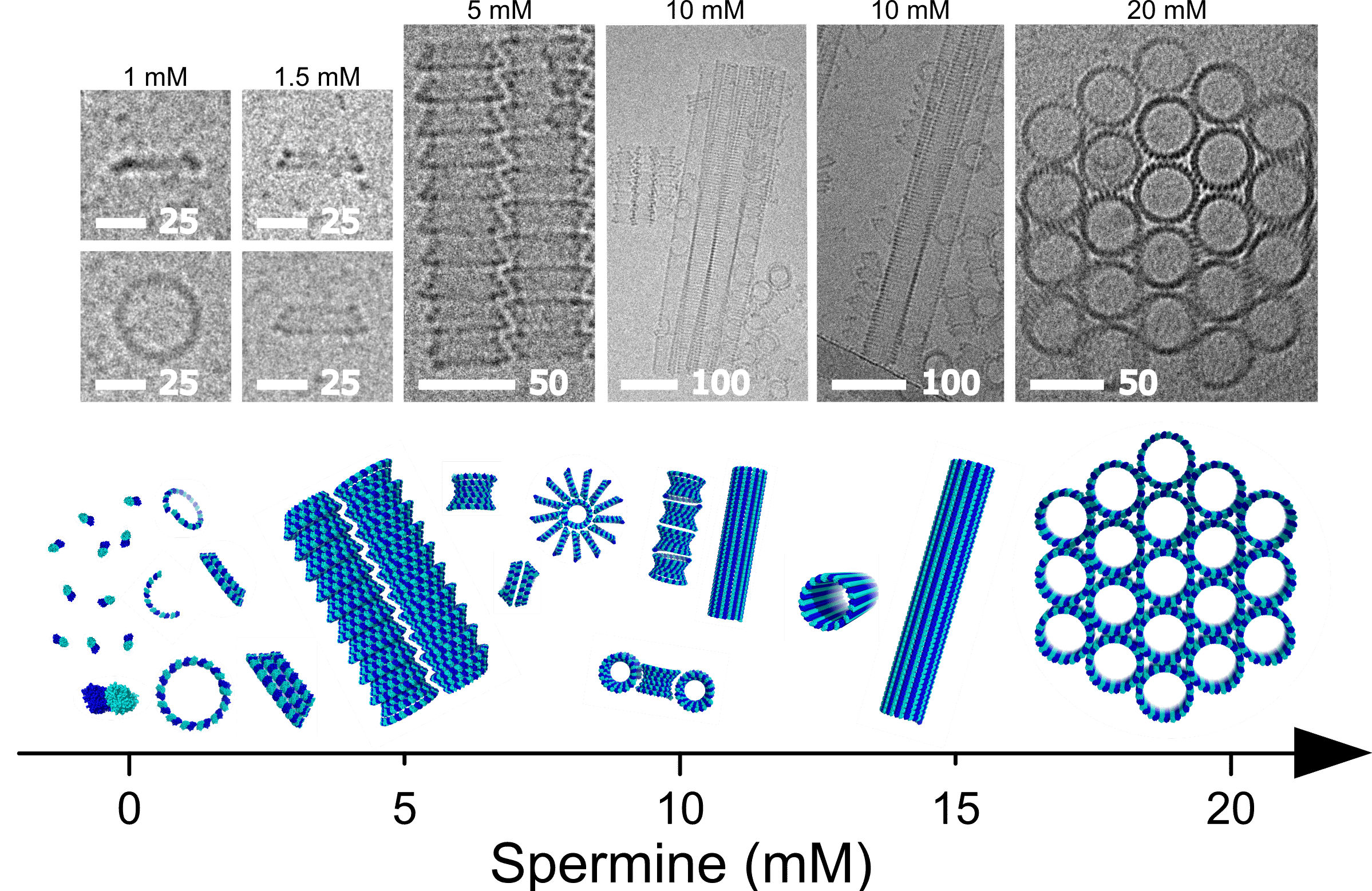
Tubulin regulates cytoskeletal activity by interacting with cellular factors and assembly into various morphologies. Spermine, an endogenous polyamine, promotes and stabilizes tubulin assemblies. Yet, the assembly structures and their formation pathways are poorly known. Here we show that spermine induced tubulin to assemble in vitro into hierarchical architectures, based on a tubulin conical-spiral (TCS) subunit. Using solution X-ray scattering and cryo-TEM, we showed that with progressive increase of spermine concentration, tubulin-dimers assembled into tubulin helical-pitch, TCSs, TCS tubes, antiparallel bundles of TCS tubes in a quasi-hexagonal symmetry, and eventually hexagonal bundles of inverted tubulin tubules. Time-resolved experiments revealed that tubulin assemblies formed at low spermine concentrations were precursors of the assemblies formed at higher spermine concentrations. The results provide insight into the regulatory role that spermine may have on the architecture of cytoskeletal networks, and contribute to the understanding of fundamental interactions that control the composition and construction of protein-based biomaterials.
Powered by Eventact EMS