
Nickel-catalyzed cross-coupling reactions for the synthesis of benzyl fluoride derivatives
Fluorinated organic compounds have been utilized in many pharmaceutical and agricultural applications.[i] Therefore, selective fluorination of organic molecules has become a field of great interest. In this work, we focus on fluorinated organic molecule that contain a fluorine atom in the benzylic position, since this position is biologically active.[ii]
Nickel-catalyzed cross-coupling reactions using fluoro-halo-compounds as starting materials is an efficient synthetic approach to prepare fluorinated organic compounds.[iii]
Here we present a nickel catalyzed Suzuki C(sp3)-C(sp2) cross-coupling reaction for the synthesis of diaryl-fluoroides and a nickel-catalyzed C(sp3)-C(sp3) Negishi cross-coupling reaction for the synthesis of benzyl fluoroalkanes, utilizing the same substrate, benzyl fluorobromides, as an electrophile.
([i]) (a) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly, and N. A. Meanwell J. Med. Chem. 2015, 58, 8315. (b) S. Purser, P. R. Moore, S. Swallow, and V. Gouverneur Chem. Soc. Rev., 2008, 37, 320.
([ii]) (a) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Acena, V. A. Soloshonok, K. Izawa, and H. Liu Chem. Rev. 2016, 116, 422. (b) M. M. Bio, M. Waters, G. Javadi, Z. J. Song, F. Zhang, D. Thomas. Synthesis 2008, 6, 891.
([iii]) (a) X. Jiang, S. Sakthivel, K. Kulbitski, G. Nisnevich, and M. Gandelman J. Am. Chem. Soc. 2014, 136, 9548. (b) X. Jiang and M. Gandelman J. Am. Chem. Soc. 2015, 137, 2542. (c) J. Sheng, H. Q. Ni, G Liu, Y. Li, and X. S. Wang Org. Lett. 2017, 19, 4480.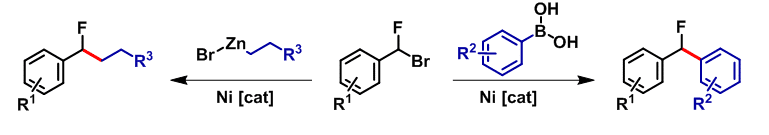
Powered by Eventact EMS