
Novel triazatriangulenium cationic membranes for fuel cell applications
2Department of Chemistry, St. Berchmans College, Changanassery, Kottayam, Kerala, India
3International and Inter University Center for Nanoscience and Nanotechnology, Mahatma Gandhi University, Changanassery, Kottayam, Kerala, India
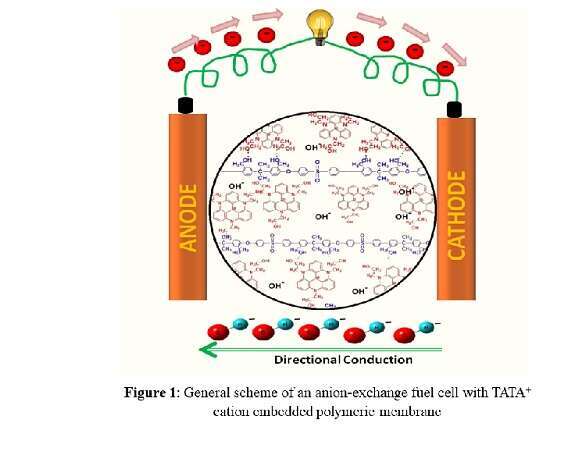
Fuel cells are perceived as the environment-friendly energy sources of choice for the 21st century. The principle behind fuel cells is to convert energy stored in chemical bonds to generate electricity and produce water as waste. Polymer-electrolyte-membrane-based alkaline fuel cells have been designed for clean, cost effective and efficient energy conversion devices for stationary, automotive, and portable power applications.The Anion exchange membrane (AEM) is the critical component in AEM fuel cells and its function is to transport hydroxide anions from the cathode to the anode. The major challenge for the commercialization of AEM fuel cells is to produce membranes with appropriate chemical and physical stability, suitable mechanical properties, as well as excellent hydroxide conductivity. Herein, we introduce a polysulfone (PSF) based AEM non-covalently embedded with triazatriangulenium1,2 (TATA+) cations. The pore filling effect of TATA+ in the PSF membrane was confirmed by High-Resolution Scanning Electron Microscopy (HRSEM) . The ionic conductivity of these membranes are 4 to 7 times higher than the pristine membranes in the 25-80 ℃ range. Alkaline stability analysis shows high retention of ion conductivity as observed for samples in 2 M KOH at 60 °C over 30 days. Thermogravimetric analysis also indicates the excellent thermal stability of our membranes. Our findings will contribute to the development of convenient TATA+ based membranes for alkaline fuel cell applications.
References
- Laursen, B.W.; Krebs, F.C. Chem.: Eur. J. 2001, 7(8), 1773-1783.
- Kim, Y.; Wang, Y.; France-Lanord, A.; Wang, Y.; Wu, Y.-C. M.; Lin, S.; Li, Y.; Grossman, J. C.; Swager, T. M. J. Am. Chem. Soc. 2019, 141(45), 18152-18159.
Powered by Eventact EMS