
Development of phosphothreonine as a catalytic residue in peptide-mediated asymmetric transformations
Chiral phosphoric acids (CPAs) have become prolific organocatalysts for a plethora of asymmetric transformations; however, departure from the prevalent BINOL-derived scaffolds is limited. Recent work in the Miller group has found that phosphothreonine-(pThr)-embedded peptides constitute a new class of chiral phosphoric acids (CPAs), enabling highly site- and enantioselective chemical transformations. Herein, we report the rational design of a multifunctional pThr-catalyst for the enantioselective Baeyer–Villiger oxidation of cyclobutanones. The functional diversity of the peptidic scaffold provides the opportunity for multiple points of contact with substrates via hydrogen bonding, and the ease of peptide synthesis facilitates rapid diversification of the catalyst structure. Utilizing a hypothesis-driven design, we identified a pThr-based catalyst that contains an N-acylated diaminopropionic acid (Dap) residue, which achieves high enantioselectivity with low catalyst loadings (0.5–2.5 mol%). The power of peptide-based multisite binding is exemplified through reversal in the absolute stereochemical outcome upon repositioning of the substrate−directing group, and modifications to the i+3 residue (LDap to LPhe) lead to an observed enantiodivergence. Structure−selectivity and 1H−1H-ROESY studies revealed the proposed hydrogen bonding interactions are essential for high enantioinduction. The ability for the phosphopeptides to operate as multifunctional oxidation catalysts expands the scope of pThr catalysts and provides a framework for the future selective diversification of more complex substrates, including natural products.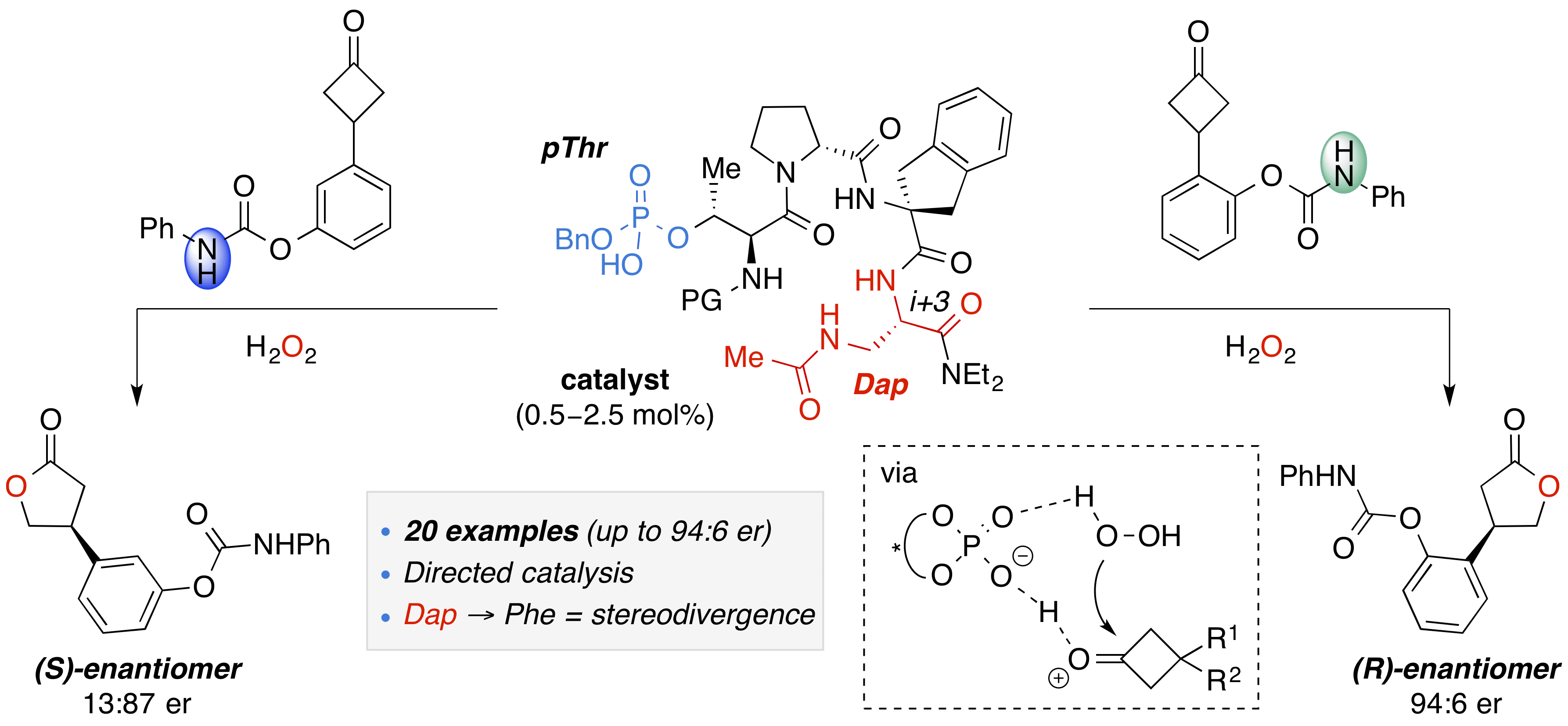
Powered by Eventact EMS