
Inverse double bond reactivity in olefin metathesis reactions of highly strained dicyclopentadiene derivatives
Anionic ligands in Ruthenium catalysts have a crucial role in determining olefin metathesis activity.1 In particular, we have observed a unique result with a Ruthenium diiodo sulfur chelated complex (Ru-SPh-I) (Scheme 1).2 This catalyst showed very good efficiency in ring closing metathesis (RCM) reactions, while it was completely inert towards ring opening metathesis polymerization (ROMP).1
However, it has been reported that the dichloro trifluoromethylsulfur-chelated Ruthenium (Ru-SCF3-Cl) catalyst has one of the best activities towards olefin metathesis reactions, due to its fast initiation when using heat or light irradiation.
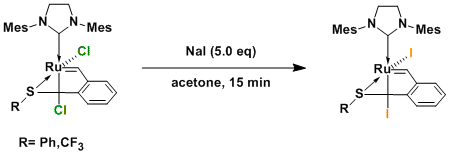
In order to further understand the effect of the iodide ligands we decided to investigate also Ru-SCF3-Cl and thus exchanged the anionic ligands from chlorides to iodides.
Herein, we report the successful synthesis of Ru-SCF3 -I. However, the reactivity of the SPh chelated complex and SCF3 chelated complexes were quite different. With the new complex, dicyclopentadiene (DCPD) derivatives were reactive when mixed with DEDAM. After careful analysis we determined that only the less strained (but less hindered!) cyclopentene ring was reacting; while the norbornene ring remained untouched. Typically, DCPD polymerizes easily with Ru catalysts, but by using our new complex we can obtain a single molecule with inverse selectivity. This new reactivity opens the path for the synthesis of novel molecules, which could not be made before (or would require extensive synthetic efforts).
[1] Ivry, E., Nechmad, N.B., Baranov, M., Goldberg, I. and Lemcoff, N.G., Inorg. Chem., 2018, 57, 15592-15599.
[2] Noy B. Nechmad, Ravindra Phatake, Elisa Ivry, Albert Poater, and N. Gabriel Lemcoff, Submitted.
Powered by Eventact EMS