
Bifunctional nanoscale assemblies: Multistate electrochromics coupled with charge trapping and release
We demonstrate charge transport, storage and release in thin films and devices by utilizing a layer of well-defined ruthenium based complex as an electron-transport gate that opens and closes in response to a voltage. These electrochemical processes are reversible and are accompanied by distinct color-to-color transistions (red, bordeaux, brown, and transparent). The heterojunction of 320 nm thick consist of the nanoscale gate bound to a flexible and transparent electrode (ITO) and a charge storage layer attached to the surface of the gate. The charge storage layer consist of isostructural iron complexes. Electrochemically varying the oxidation states of the ruthenium complexes opens up electron‐transport channels that allows controlled addressing of the oxidation states of iron complexes; hence storage and release of positive charges. The charge storage is mediated by trivalent ruthenium complexes whereas the discharging process is mediated by monovalent complexes. This work have been accepted for publication as a communication in Angewandte Chemie.
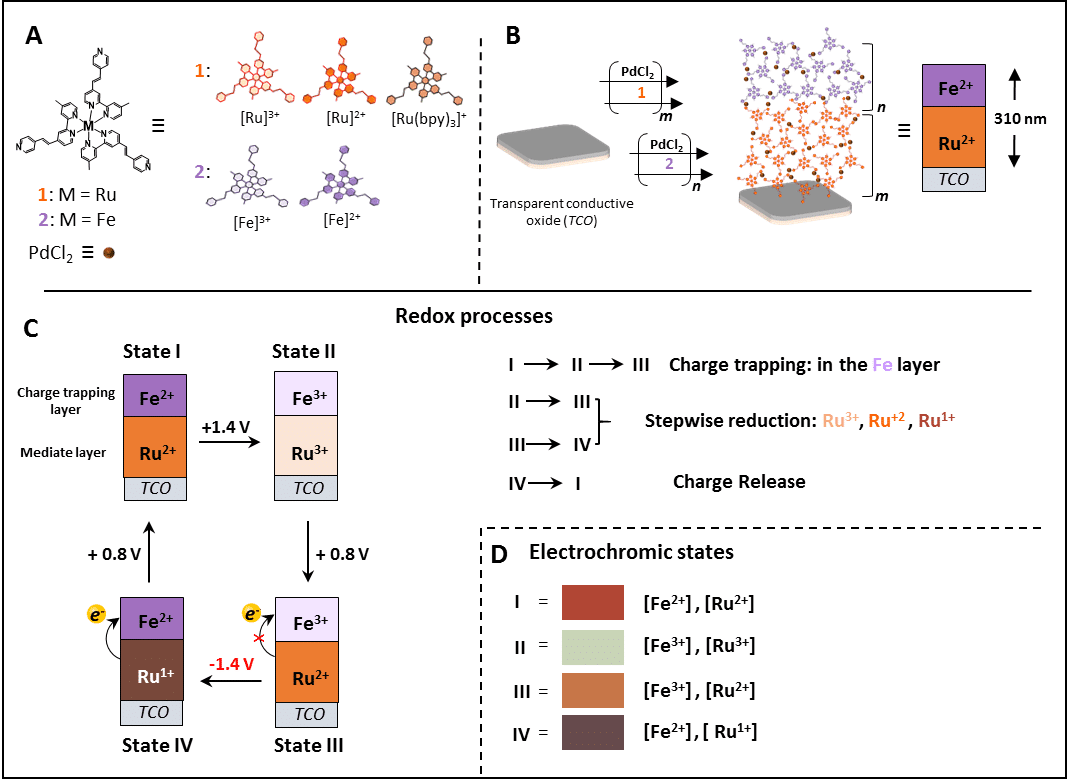
Metallo-organic bilayers, their redox, and electrochromic properties. A) Molecular components used for the formation of the nanoscale metallo-organic assemblies. B) Surface assembly sequence: m = the number of deposition steps for PdCl2(PhCN)2/1, n = the number of deposition steps for [PdCl2(PhCN)2/2]. C) The different states of the bilayer. D) Color and oxidation states of the bilayers.
Powered by Eventact EMS