
Divergent chemical transformation of alcohols as renewable raw material
Mohamed Agbaria, Amar Mohite, N. Gabriel Lemcoff
Halogen and amine-containing organic compounds have numerous industrial and practical uses1,2 and also appear in thousands of natural products.3 For instance, several organic halides are produced in large scale and used as feedstocks for PVC production, pharmaceuticals, synthesis of pesticides, herbicides and commercial fertilizers, just to name a few of their uses.4
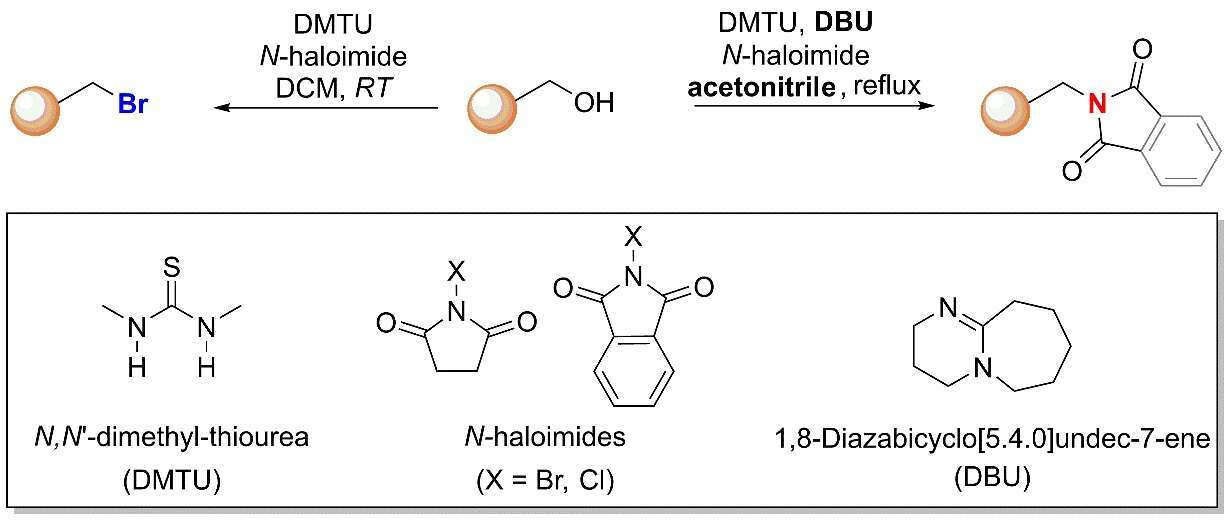
Previously, we have demonstrated the bromination or chlorination of alcohols facilitated, by sub-stoichiometric amounts of thiourea additives with N-halosuccinimide (NHC) as halogen donor source. This reaction was found to be useful for all types of alcohols, including primary, secondary tertiary and unsaturated alcohols. The reaction proceeds in non-polar organic solvents under very mild conditions without the need of alcohol pre-activation. The succinimide byproduct may be recycled by halogenation, making the method extremely atom efficient and amenable for large-scale processes in industry and academia.
In this study, we show that addition of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) to the reaction mixture afforded an imide product which is easily converted to amine following treatment with hydrazine. Thus, we developed selective divergent of organic halides and amine derivatives by using renewable alcohol starting material in a very efficient and convenient manner.
references
- a) D. A. Petrone, J. T. Ye, M. Lautens Chem. Rev. 2016, 116, 8003-8104; b) I. Saikia, A. J. Borah, P. Phukan Chem. Rev. 2016, 116, 6837-7042
- Lawrence, S. A. Amines: Synthesis, Properties and Applications; Cambridge University Press: 2004; 450 pp.
- 3. a) G. W. Gribble “Naturally occurring organohalogen compounds - A comprehensive update” in series “Progress in the Chemistry of Organic Natural Products”, Springer-Verlag, Wien, 2010, Vol 91;
- a) P. Jeschke, W. Krimer, U. Schirmer, M. Witschel “Modern Methods in Crop Protection Research”, Wiley-VCH (first edition), 2012
Powered by Eventact EMS