
Invited
Drawing on the secondary sphere of organocatalysts to control reaction mechanisms
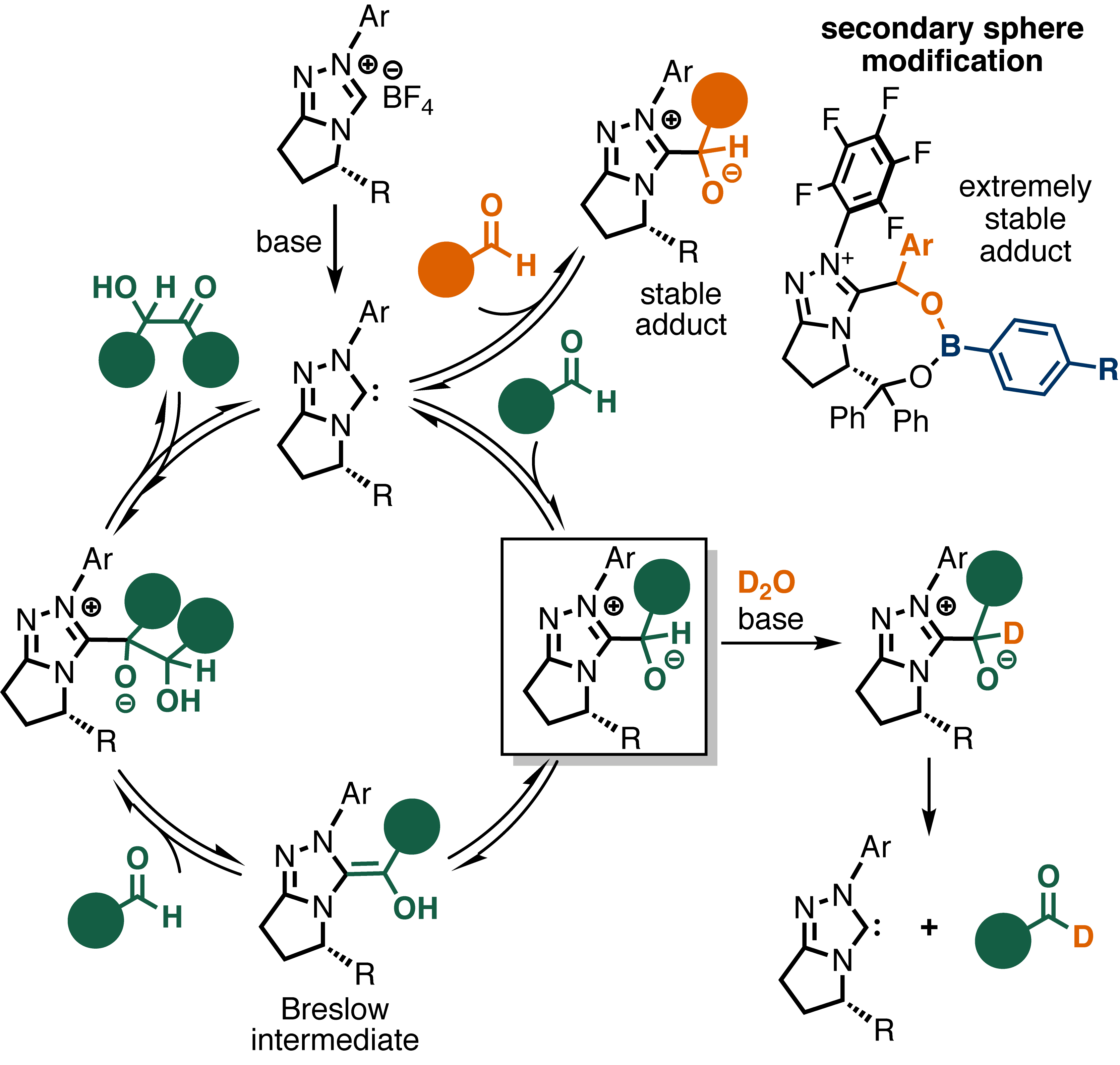
Our group has been involved in the in situ modification and mechanistic interrogation of secondary-sphere interactions in organocatalysis. As a proof-of-concept, we modified the secondary shpere of N-heterocyclic carbenes (NHCs) using boronic acids (BAs) under reaction conditions and tested their reactivity and selectivity in the benzoin reaction. This strategy allowed us to control the selectivity of the benzoin reaction and to probe its mechanism. Kinetic studies and computations revealed the different pathways this reaction follows with and without a secondary sphere modifier. By studying the mechanism we were in the position to intercept reactive intermediates, which allowed us to identify a mild selective aldehyde deuteration reaction.
Powered by Eventact EMS