
The Efficacy and Safety of Intraarticular Injection of Triamcinolone Acetonide Versus Triamcinolone Hexacetonide in the Treatment of Juvenile Idiopathic Arthritis
2Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel, ישראל
3Pediatric Rheumatology Unit, Schneider Children's Medical Center of Israel, Petach Tikva, Israel, ישראל
4Pulmonary Institute, Schneider Children's Medical Center of Israel, Petah Tikva, Israel, ישראל
5Department of Pediatrics B, Schneider Children’s Medical Center of Israel, Petah Tikva, Israel, ישראל
Background and objective: Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease of childhood. intra-articular corticosteroids joint injection (IAJI) with Triamcinolone hexacetonide (TH) or triamcinolone acetonide (TA) is considered as the first-line therapy for oligoarticular JIA. Our unit has experience with both regimens and therefore we aimed to compare the efficacy and safety of TA versus TH for oligo and poly-articular JIA patients.
Methods: Chart review of JIA patients who were randomly (based on drug availability) treated with TA or TH IAJI between 2010-2019 was conducted. The primary outcomes for efficacy and safety were defined as full recovery of arthritis and occurrence of adverse events (AEs) one month after IAJI respectively.
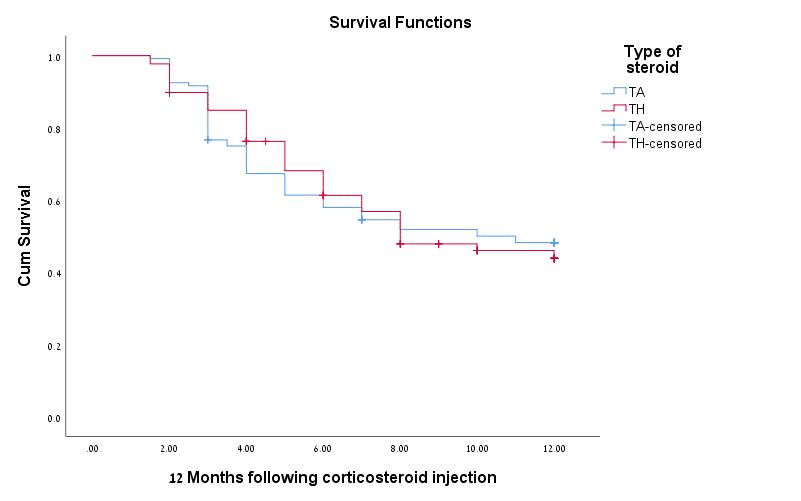
Results: Overall 239 joints of 80 JIA patients were treated (136 TA/103 TH joints). Complete recovery was documented in 69.1% TA and 71.8% TH groups. 8.8% TA and in 11.7% treated patients had no response or worsening of arthritis (p=0.485). No AEs were documented except of minor scar at 2 joints injection sites.
Conclusion: We demonstrated complete recovery of arthritis in about 70% of IAJI treated patients and similar efficacy of TA and TH regimens along with high safety profile. These findings are specifically important due to contemporary shortage of TH in the US market.
Powered by Eventact EMS