
Invited
1D and 2D materials for catalyst protection
Hydrogen-bromine redox-flow batteries (RFBs) technology offers the most economic storage solution and is considered most promising for a sustainable electricity storage solution due to its fast kinetics, highly reversible reactions and low chemical costs. The main bottleneck of conventional electrodes is the rapid fading of the hydrogen catalyst performance in the highly corrosive environment.
The crossover of Br species requires the use of a catalyst with a high PGM (platinum group metals). To increase the effectiveness of the storage system, the catalyst cost needs to be decreased (by reducing the PGM loading) whilst increasing its tolerance versus bromide species. A possible solution to this problem is a selective coating on the surface of the catalyst. The common examples include organic polymers and oxides. Carbon 1D and 2D materials, such as carbon nanotubes or graphene, may provide another pathway to protecting the catalyst. Such materials provide an extremely thin coating, which should be block large species from reaching the catalyst without hindering hydrogen/protons.
We show that a coating on the catalyst can effectively protects the metallic surface from corrosion in concentrated HBr and maintains a high catalytic activity.1-3
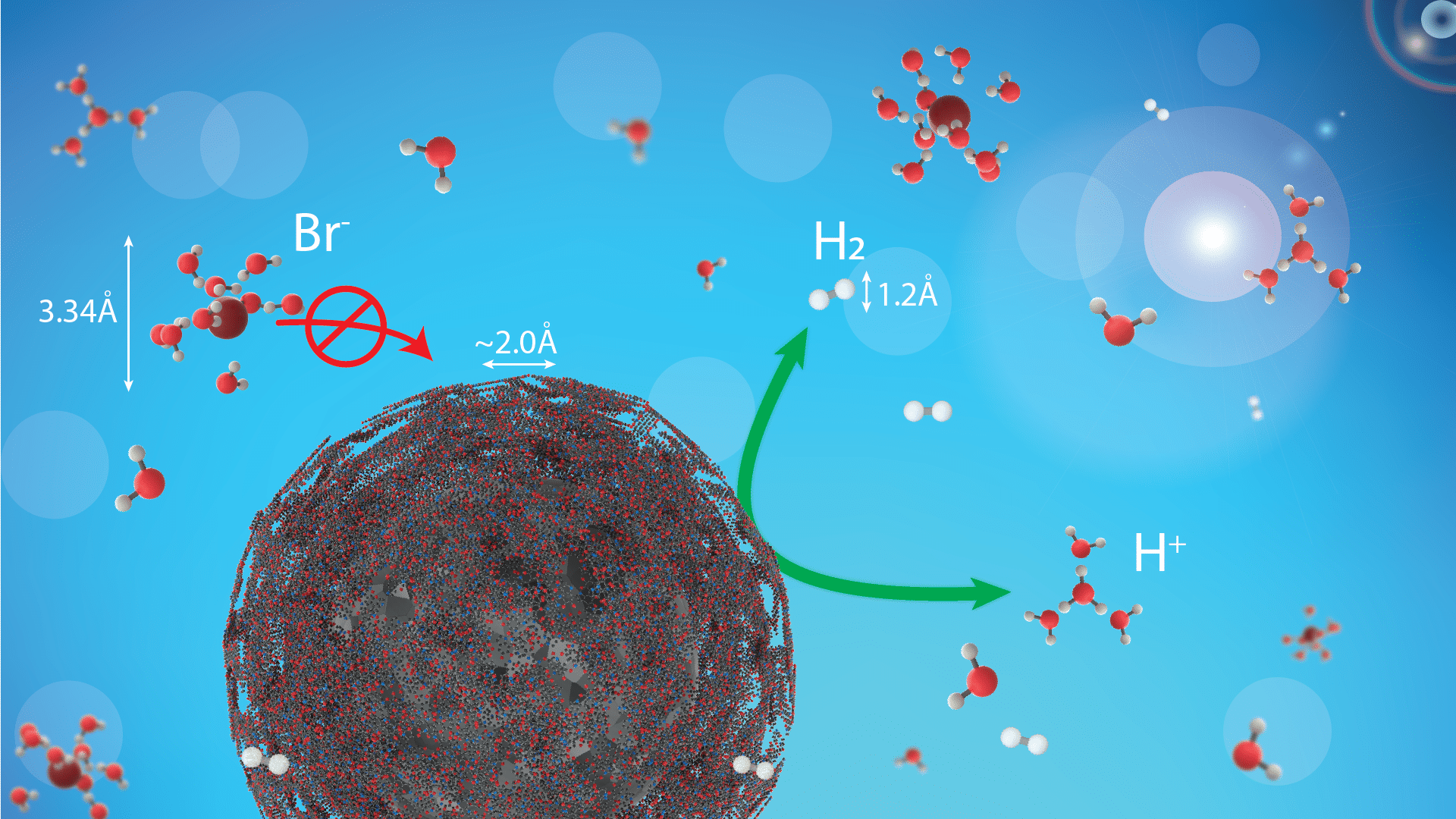
Figure: Illustration of the protective coating on the catalyst nanoparticle which allows a free diffusion of hydrogen species and blocks bromide species
References:
- PCT Application No. PCT/IL2018/050254
- From the Sea to Hydrobromic Acid: Polydopamine Layer As Corrosion Protective Layer on Platinum Electrocatalyst ACS Appl. Energy Mater., 2018, 1(9), pp 4678–4685
- Crossover-Tolerant Coated Platinum Catalysts in Hydrogen/Bromine Redox Flow Battery J. Power Sources 2019, 422, 84
Powered by Eventact EMS