
A Host-signature Test Improves Aaccuracy of Bacterial vs. Viral Infection Diagnoses in Children Presenting to the Emergency Department
2בית ספר לרפואה על שם גולדמן, אוניברסיטת תל אביב, ישראל
3Memed, MeMed Diagnostics, ישראל
Background: A host-signature comprising of TRAIL, IP-10, and CRP proteins was shown to exhibit high diagnostic performance and superiority to routine biomarkers in infectious diseases. We examined its potential impact on clinician’s diagnosis of children presenting to the ED.
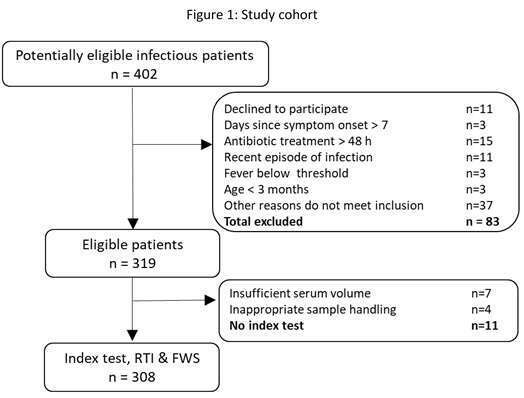
Methods: 308/463 patients aged ≥ 3 months with respiratory tract infection or with fever without source and meeting inclusion criteria were prospectively recruited at a tertiary care pediatric facility (Figure 1). History, physical examination, routine laboratory and imaging were performed; respiratory multiplex PCR and host-signature data were collected. After initial examination, attending physicians filled out a questionnaire documenting their tentative diagnoses. Reference standard labels of bacterial, viral or indeterminate infections were assigned by an expert panel unanimous adjudication based on comprehensive clinical and laboratory investigation, including a nasal swab multiplex-PCR and patient follow up. Physician’s diagnostic labels and host-signature results were subsequently compared to the reference standard. The analysis included cases with unanimous reference standard (n=233); reference indeterminates were excluded.
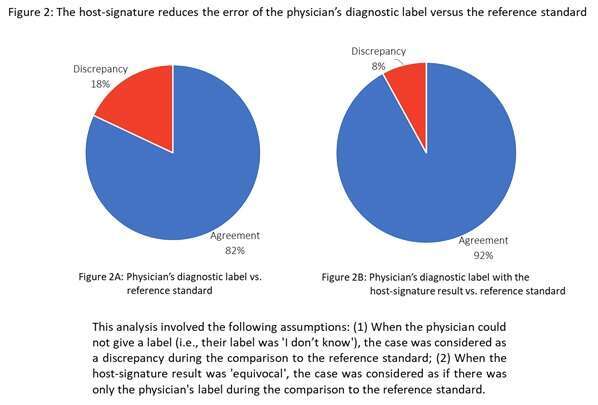
Results: Questionnaires were completed for 226/233 cases with unanimous reference standard. The physician’s label was viral for 177 cases (78%), bacterial for 37 cases (16%) and indeterminate for 12 cases (5%). As shown in figures 2A and 2B, the additional information provided by the host-signature result could have reduced the error of the physician’s label versus the reference standard from 18% to 8%.
Conclusions: This pioneer study examines the host-signature’s test performance as compared to standard-of-care. The more than 2-fold reduction in diagnostic error observed supports this tool’s potential clinical utility.
Powered by Eventact EMS