
Porphyrins – A novel scaffold for photoprotecting groups
2Department of Chemistry and Recetox Faculty of Science,, Masaryk University, Brno, Czech Republic
Photocaging1 is a widely used strategy for modulating the activity of biological systems;2 however, current photocages rely primarily on molecules that have low molar absorptivity (ε = ~500-5000 L∙mol-1∙cm-1) and require high-energy illumination1 (< 400 nm). These characteristics result in significant photodamage to biological specimens, poor tissue penetration and limited compatibility with other light-dependent techniques (optical sensors, optogenetics). The development of efficient, highly-absorbing long-wavelength excitable photocages will overcome the inherent limitations of current UV-excitable photocages. As such, I expect that they will become powerful chemical-biological tools with broad applicability in fields ranging from the synthetic and biological applications.
Herein we develop porphyrin-based photo protecting group with tunable absorption over the visible region by functionalizing methyl hydroxy moiety on the meso position. Porphyrin will provide a flexible scaffold with superb photochemical properties and inherent biocompatibility. Irradiation, kinetic and transient absorption spectroscopic analysis revealed the progress of uncaging reaction from triplet excited state of porphyrin. Metallation of porphyrin ligand help to understand metal to ligand energy or electronic transfer in photolysis reaction.3
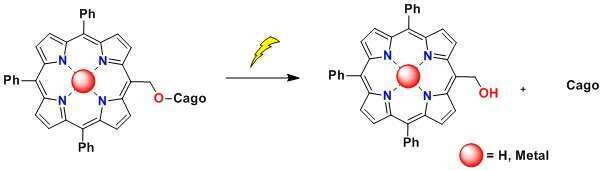
Fig. Schematic representation of photoreaction of the porphyrin photoprotecting group (PPG).
References
(1) Klan, P.; Solomek, T.; Bochet, C. G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy Chem Rev 2013, 113, 119.
(2) Shao, Q.; Xing, B. Photoactive molecules for applications in molecular imaging and cell biology Chem Soc Rev 2010, 39, 2835.
(3) Sekhar, A. R.; Chitose, Y.; Klan, P.; Weinstain, R. Manuscript under preparation.
Powered by Eventact EMS