
Improved electrochemical performance of Ni-Rich LiNi0.8Co0.1Mn0.1O2 cathode materials for Li-ion batteries upon cationic doping with molybdenum
2Institute of General and Inorganic Chemistry, Bulgarian Academy of Sciences, Sofia, Bulgaria
3Solid State Institute, Israel Institute of Technology - Technion, Haifa, Israel
4Department of Materials Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel
The development of cathode materials for Li-ion batteries (LIBs) with superior electrochemical properties – high capacity and cycling stability, low voltage fade - is key for the next generation of these power sources, especially for electric vehicles application. Ni-rich materials of layered structure LiNixCoyMnzO2, x > 0.5 are promising candidates as cathodes for advanced LIBs. The structural and cycling stability of Ni-rich cathodes can be remarkably improved by doping with small amount of extrinsic multivalent cations (Al3+, Zr4+, Ta5+, etc). Based on DFT calculations for LiNi0.8Co0.1Mn0.1O2 (NCM811) it was demonstrated in this work that Mo6+ cations preferably substitute Ni cations in the layered structure due to the lowest substitution energy compared to Li, Co, and Mn. We established that the electrochemical behavior of LiNi0.8Co0.1Mn0.1O2 as a positive electrode material for LIBs can be substantially improved by doping with 1 – 3 mol. % of Mo6+, in terms of lowering the irreversible capacity loss during the 1st cycle, increasing discharge capacity, and rate capability as shown in Figure 1a, decreasing capacity fade upon prolonged cycling, lowering the voltage hysteresis, and charge-transfer resistance. The latter is attributed to the presence of additional conduction bands near the Fermi level of the Mo-doped LiNi0.8Co0.1Mn0.1O2 (Figure 1b), which facilitate Li-ions and electron transfer within the doped material. Therefore, the charge-transfer resistance of Mo-doped electrodes is lower, as shown by impedance spectroscopy. We discuss in this presentation a unique segregation phenomenon, in which the surface concentration of the transition metals and Mo-dopant differs from that in the bulk. This near surface segregation of the Mo-dopant seems to have a stabilization effect on Ni-rich (Ni=80 at. %) cathode materials.
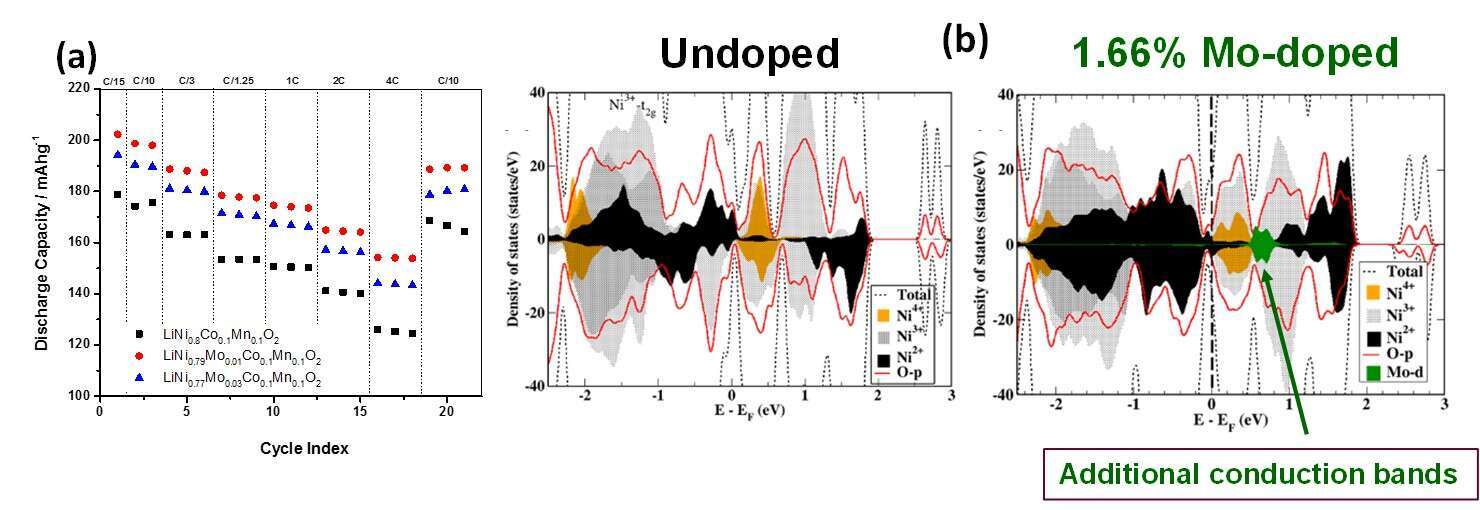
Figure 1. (a) Results of the rate capability tests of undoped and Mo-doped NCM811 materials and (b) Calculated density of states for these materials.
Powered by Eventact EMS