
A practical approach towards α-iodobimane reactive intermediates
Bimanes are a class of low molecular weight, heterobicyclic molecules presenting two isomeric forms: syn & anti.1 syn-Bimanes are generally accompanied by high quantum yield fluorescence and have found numerous uses ranging from biological fluorescent probes to laser dyes.2 Several α-halogenated bimanes derivatives have been prepared, among them the syn-(R,I)bimanes (e.g., 3) which were found to be particularly useful in C-C coupling reactions involving various acetylenes.1,3,4 The reported synthetic route for the preparation of these reactive intermediates (Fig. 1A) involves the hydrogenation of syn-(R,Cl)bimane (1) followed by the ICl iodination of the hydrogenated product (2).1
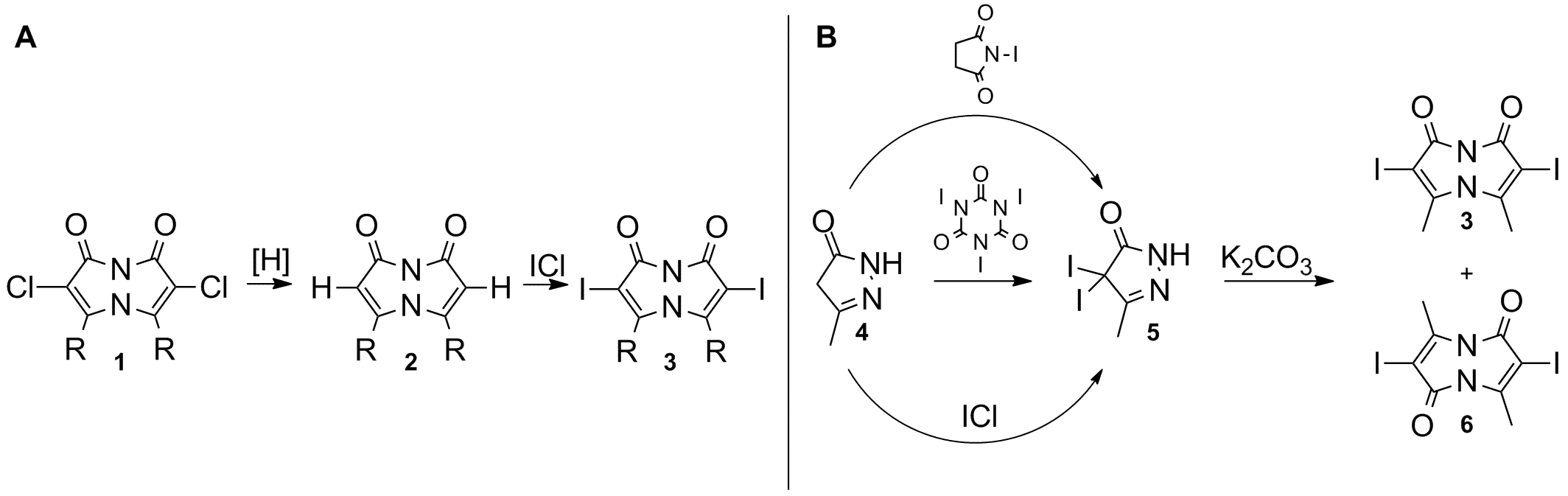
Figure 1: A) Preparation of syn-α-iodobimanes as performed by E.M. Kosower. B) Direct preparation of syn-α-iodobimanes.
In the past, the Grynszpan group reported the use of TCCA and NCS, among other chlorenium sources, in the α-chlorination of 3,4-dimethyl-2-pyrazolin-5-one, allowing easy access to the key precursor in bimane synthesis.2 We also used TCCA in the preparation of syn and anti α-chlorinated bimanes like 1 by performing double α-chlorination of 4. We reasoned that α-iodobimanes could be easily prepared using the iodo analogs of the aforementioned chlorinating agents (Fig. 1B), saving two synthetic steps.
Primary results using triiodoisocyanuric acid (TICA), indicate the low yield formation of α-iodobimanes 3 and 6. Our efforts to optimize the synthesis of α-iodobimanes will be presented.
References:
[1] Kosower, E. M.; Ben-Shoshan, M. Bimane Acetylenes and Diacetylenes. Bimanes. 33. The Journal of Organic Chemistry 1996, 61 (17), 5871–5884.
[2] Grynszpan, F.; Neogi, I.; Das, P. Dihalogen and Solvent-Free Preparation of syn-Bimane. Synlett 2018, 29 (08), 1043–1046.
[3] Kosower, E. M.; Pazhenchevsky, B. Bimanes. 5. Synthesis and Properties of syn- and anti-1,5-Diazabicyclo[3.3.0]Octadienediones (9,10-Dioxabimanes). Journal of the American Chemical Society 1980, 102 (15), 4983–4993.
[4] Szabó, T.; Bényei, A.; Szilágyi, L. Bivalent glycoconjugates based on 1,5-diazabicyclo[3.3.0]octa-3,6-diene-2,8 dione (“bimane”) as a central scaffold. Carbohydrare Res. 2019, 473, 88–98.
Powered by Eventact EMS