
Frustrated Lewis pairs comprising nitrogen Lewis acids for Si-H bond activation
Frustrated Lewis Pairs (FLP) were discovered in 2007 by Stephan and Erker. They reported the activation of a hydrogen molecule by a non-metallic species.1-2 Therefore, they used molecules containing both an electron-accepting boron atom, and a bulky electron-donating phosphine. Despite having a significant driving force for bond formation, these two moieties do not form a Lewis adduct because of steric congestion. The subsequent "frustration" renders them available for chemical bond activations.
Recently, we have demonstrated an unprecedented Lewis acidic behavior of nitrenium cations.3 It represents the first examples of nitrogen-based Lewis acids. In contrast to common Lewis acids used in FLPs, which utilize the empty orbital of a boron atom, nitrogen Lewis acids utilize an electrophilic nitrogen atom. Considering the superior electronegativity of nitrogen compared with boron, these molecules have the potential to activate a plethora of chemical bonds.
We have recently synthesized4 a new nitrenium-based Lewis acid which effectively cleaves Si-H bonds in a quantitative yield. The generated triazane was fully characterized in a solution by multinuclear NMR techniques and in the solid state by single crystal X-ray crystallography. To the best of our knowledge, this represents a first example of a FLP consisting of a nitrogen-based Lewis acid, capable of cleaving an electrophile-hydride (E-H) type bond. Attempts to utilize this FLP for catalytic hydrosilylation are underway.
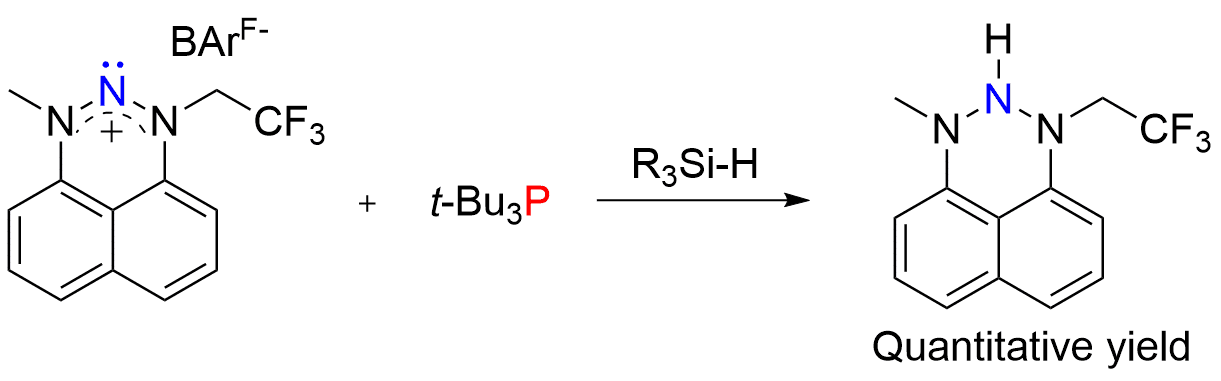
Figure – Si-H bond activation by our nitrogen-based FLP
References:
- G. C. Welch, R. R. San Juan, J. D. Masuda, D. W. Stephan, Science 2006, 314, 1124-1126.
- P. Spies, G. Erker, G. Kehr, K. Bergander, R. Fröhlich, S. Grimme, D. W. Stephan, Chem. Commun. 2007, 2, 5072-5072.
- A. Pogoreltsev, Y. Tulchinsky, N. Fridman, M. Gandelman, J. Am. Chem. Soc. 2017, 139, 4062-4067.
- I. Avigdori, A. Pogoreltsev, M. Gandelman, Manuscript in preparation.
Powered by Eventact EMS