
Deuteration of functionalized aromatic aldehydes by N-Heterocyclic Carbene (NHC) organocatalysis
Deuterated compounds enable absorption, distribution, metabolism, and excretion (ADME) studies of drug molecules, as well as the study of reaction mechanisms.1 The prevalence and utility of aldehydes in synthesis mark them as ideal candidate for the incorporation of deuterium labels. However, the common procedures for obtaining deuterioaldehydes entail the use of reagents such as LiAlD4, deuterated Schwartzs reagent (Cp2ZrDCl), D2 gas with iridium catalysts, or D2O with Ru catalysts. These approaches are limited in their practical applications due to functional group sensitivity, low reagent availability, and poor deuterium incorporation. We recently modified the secondary sphere of N-Heterocyclic Carbene (NHC) catalysts using boronic acids (BA) to afford highly selective benzoin reactions.2 Mechanistic studies revealed that the BA stabilize the the acyl anion and Breslow intermediates in the benzoin reaction. In this work, we capitalized on the reversibility of the benzoin reaction and the relative stability of the acyl anion adducts. By further stabilizing these adducts we were able to fully suppress the formation of the benzoin product and in the presence of D2O produce deuterioaldehydes. This approach afforded highly selective reactions with excellent yields and deuterium incorporation, thus providing a reliable method for late stage deuteration.
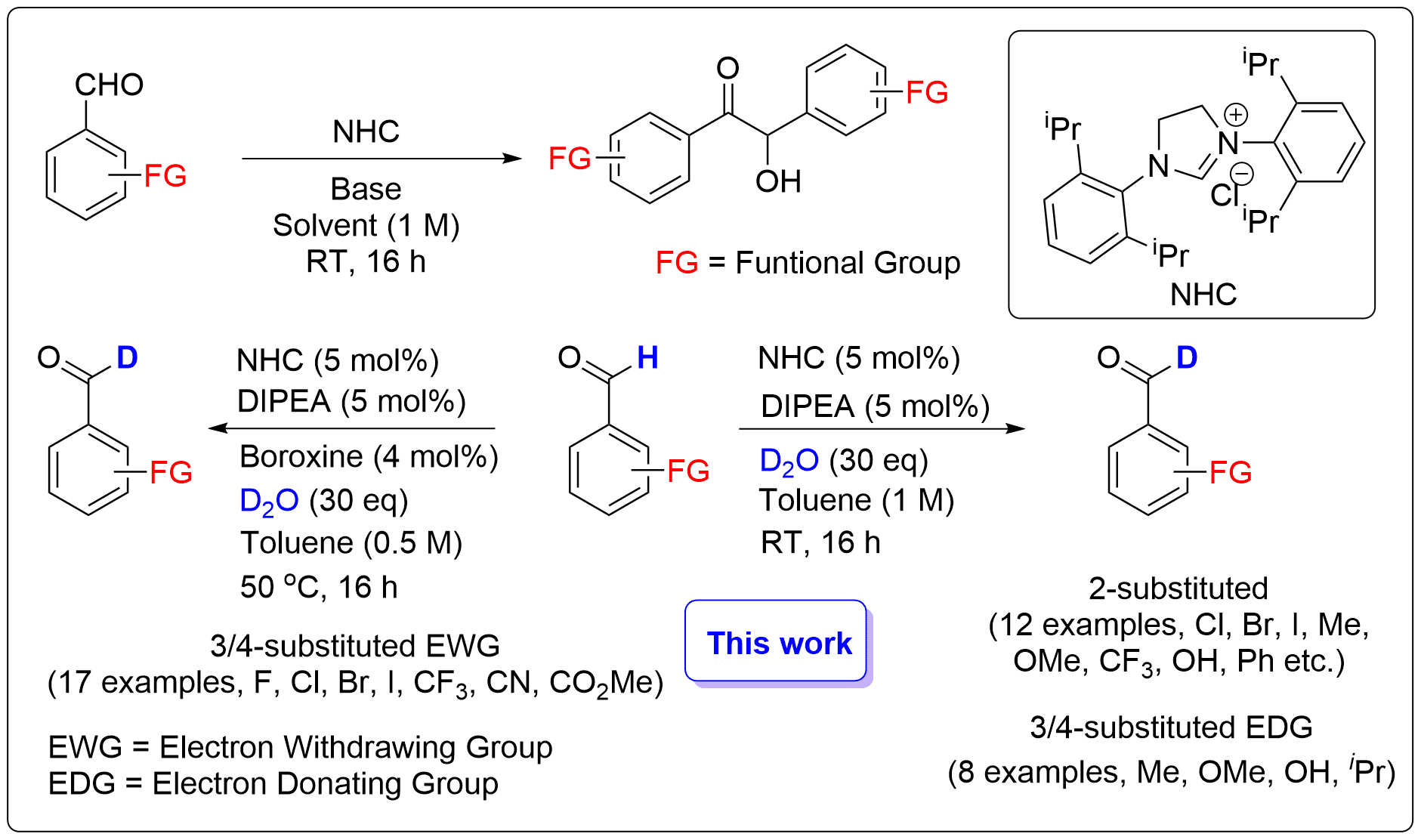
Figure 1. Synthesis of functionalized deuterated aromatic aldehydes
References
- 1. Loh Y. Y. et al., Science, 2017, 1182-1187.
- Dhayalan, V. et al., Nature Chemistry, 2019, 11, 543-551.
Powered by Eventact EMS