
Reversible Fetal Tracheal Occlusion in Mice: A Novel Minimal Invasive Technique
2Department of Pediatric Surgery, School of Medicine, Koc University, Istanbul, Turkey
3Koc University Research Center for Translational Medicine (KUTTAM), Koc University, Istanbul, Turkey
Background: There is a certain need for reversible, cheap, and reproducible animal models for understanding the impact of tracheal occlusion (TO) in congenital diaphragmatic hernia (CDH) and pathophysiology. We aimed to define an easy, reversible, and minimally invasive murine TO model, which will be useful in defining how TO impacts lung molecular biology, cellular processes, and overall lung physiology both in normal and CDH lungs.
Methods: Time-mated C57BL/6 mice underwent laparotomy at embryonic day 16.5 (E16.5) with transuterine TO performed on two fetuses in each uterine horn. While in TO group the fetuses harvested at E18.5 without suture removal; the suture was released at E17.5 in TO-R group and all fetuses harvested at E18.5. The lungs of the fetuses were compared by morphometric and histologic analysis.
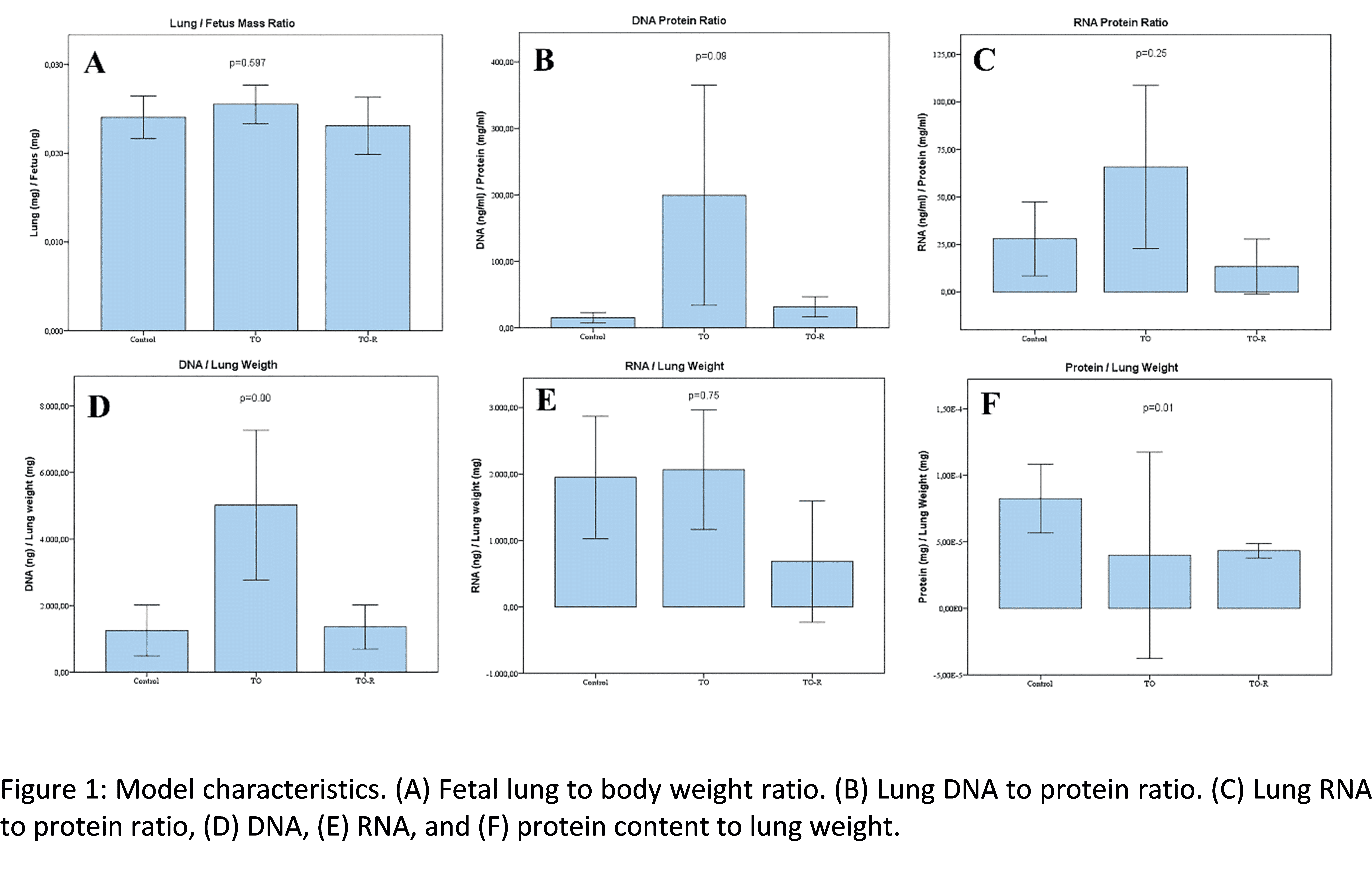
Results: Successful TO was confirmed in 34 of 37 fetuses. Twentynine of them survived to E18.5 (90.6%), 6 of the fetuses had a spontaneous vaginal delivery. Fetal weights were comparable, but there was significant difference in lung weights and lung to body weight ratios (0.020 ± 0.006 vs. 0.026 ± 0.002 vs. 0.023 ± 0.005, p=0.013). DNA/protein and DNA/lung weight ratios were elevated while protein/lung weight ratio was lower in TO compared to control and TO-R groups.
Conclusions: Reversal of fetal transuterine TO in mice is feasible with comparable outcomes to other current animal models with certain advantages and potential to translate the studies to the human.
Powered by Eventact EMS