
The Effect of Faecal Calprotectin Testing on the Referral Rate of Children with Chronic Gastrointestinal Symptoms in Primary Care: Protocol for A Randomised Controlled Trial
2Department of Paediatric Gastroenterology, University Medical Center Groningen, Netherlands
3Department of Epidemiology, University Medical Center Groningen, Netherlands
Background: Children with chronic gastrointestinal symptoms are frequently seen in primary care, yet general practitioners (GPs) often experience challenges distinguishing functional gastrointestinal disease (FGID) from somatic disorders. Inflammatory bowel disease (IBD), among others, needs to be ruled out before diagnosing FGID. Previous studies showed a high diagnostic accuracy of faecal calprotectin (FCal) for IBD in children in primary care.
Objective: To evaluate whether a test strategy that includes FCal point-of-care-testing (POCT) can reduce the referral rate to paediatric specialist care among children with chronic gastrointestinal symptoms.
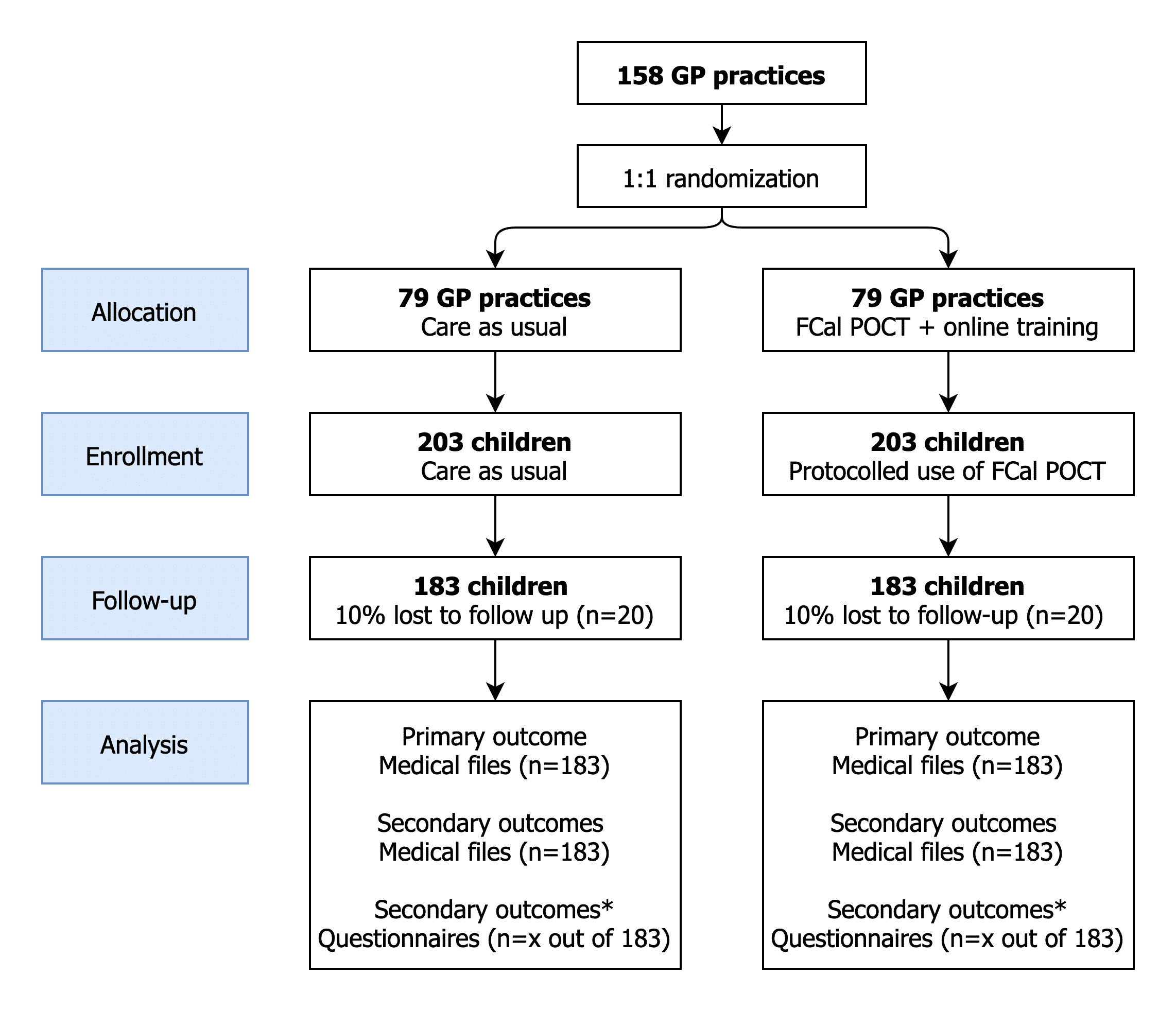
Methods: In this pragmatic cluster randomised controlled trial, we will randomise GP practices into intervention and control groups (Figure 1). The intervention group will use FCal POCT when indicated, after completing an online training about its indication, interpretation, follow-up and communication about FGID. The control group will provide care as usual according to Dutch GP guidelines that advise against FCal testing in children. GPs will include children aged 4–18 years presenting in primary care with chronic diarrhoea or recurrent abdominal pain. The primary outcome will be the referral rate for children with chronic gastrointestinal symptoms within 6 months after this assessment. Secondary outcomes, including parental concerns and satisfaction, gastrointestinal symptoms, symptom impact on daily functioning, quality of life, the proportion of children with paediatrician-diagnosed FGID, health service use and costs, will be evaluated by questionnaires completed at baseline and at 3- and 6-months’ follow-up. A sample size calculation indicates that we need to recruit 158 GP practices to recruit 406 children. We will enroll GP’s and patients from October 2019 until October 2021. By September 2020, 158 GP practices have joined the study, and 240 patients have been recruited.
Conclusion: This impact study is an essential but frequently omitted step in evaluating the utility of FCal in children in primary care.
Powered by Eventact EMS