
The Potential Coverage of Polysaccharide Vaccines for Invasive GBS Disease in Young Infants in The Netherlands (1987-2019)
2Department of Medical Microbiology and Infection Prevention, Amsterdam UMC, Amsterdam, Netherlands
3Department of Medical Microbiology and Infection Prevention, Netherlands Reference Laboratory for Bacterial Meningitis, Amsterdam, Netherlands
4Department of Pediatrics, Amsterdam UMC, Amsterdam, Netherlands
Background: Maternal immunization is a promising way to reduce Group B Streptococcus (GBS) meningitis and sepsis in young infants. Trivalent (serotypes Ia, Ib, III), pentavalent (Ia, Ib, II, III, V) and hexavalent (Ia, Ib, II, III, IV, V) vaccines against GBS were shown to be safe and immunogenic in phase 1 and 2 studies.[1]
Objective: We present the potential long-term coverage of these vaccines in the Netherlands.
Methods: Nationwide surveillance data from the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) was used to identify GBS isolates cultured in blood or cerebral spinal fluid (CSF), in infants aged 0-3 months, between 1987-2019. The NRLBM receives approximately 85% of these isolates.[2] Serotyping with latex agglutination was performed. Potential vaccine coverage was calculated based on serotype prevalence. The Chi-square test was used to compare potential vaccine coverage in different time periods and between sepsis and meningitis episodes.
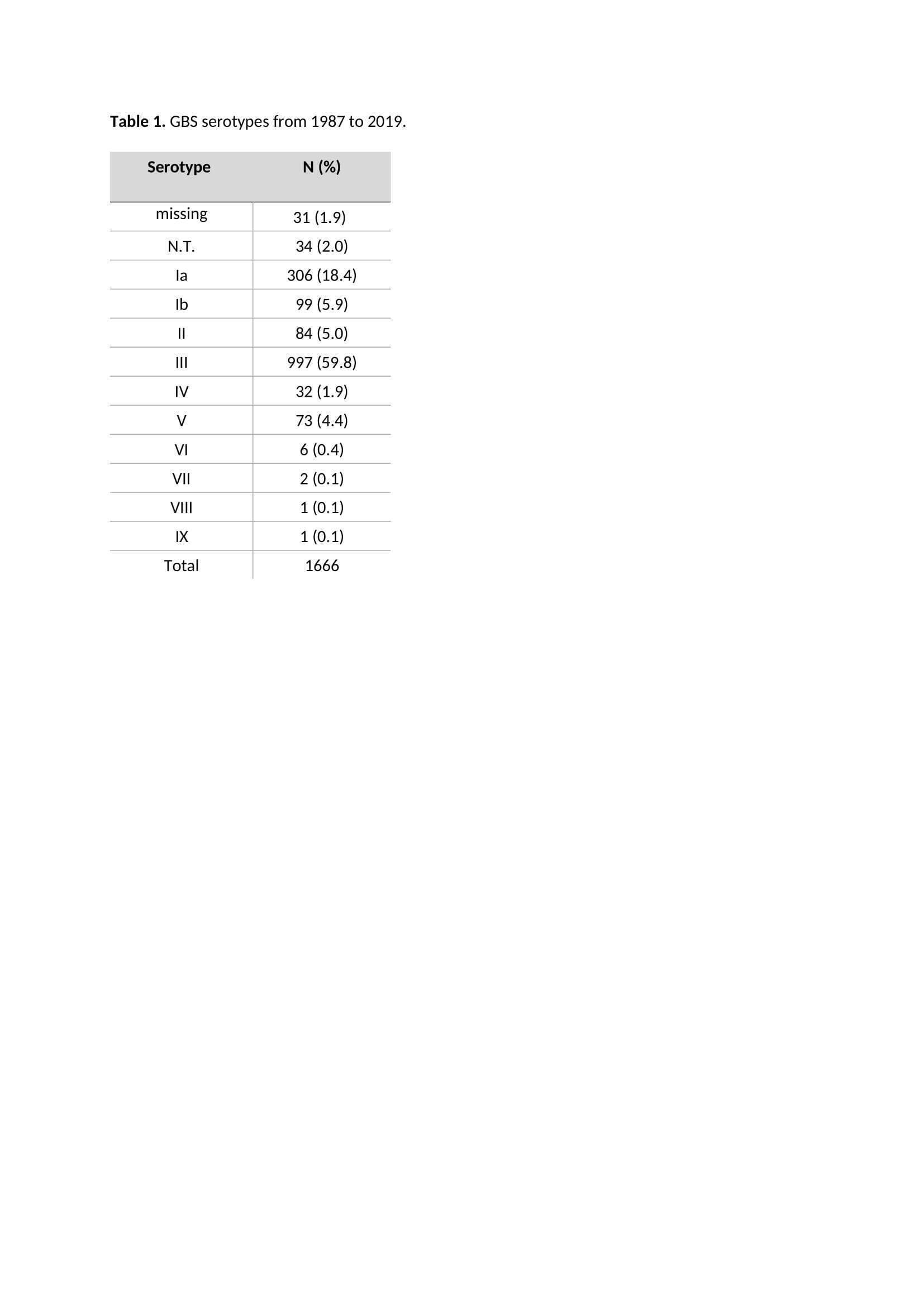
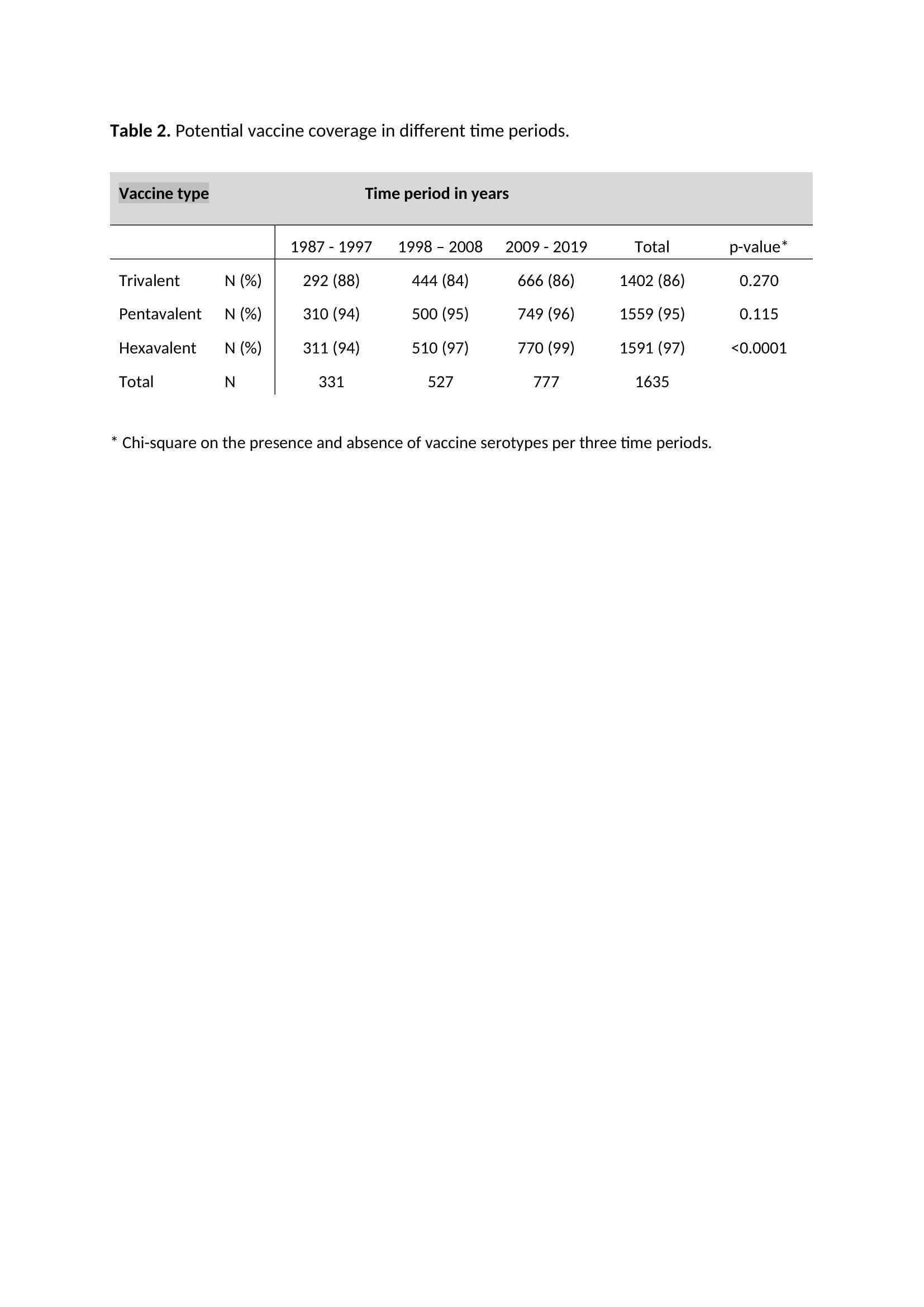
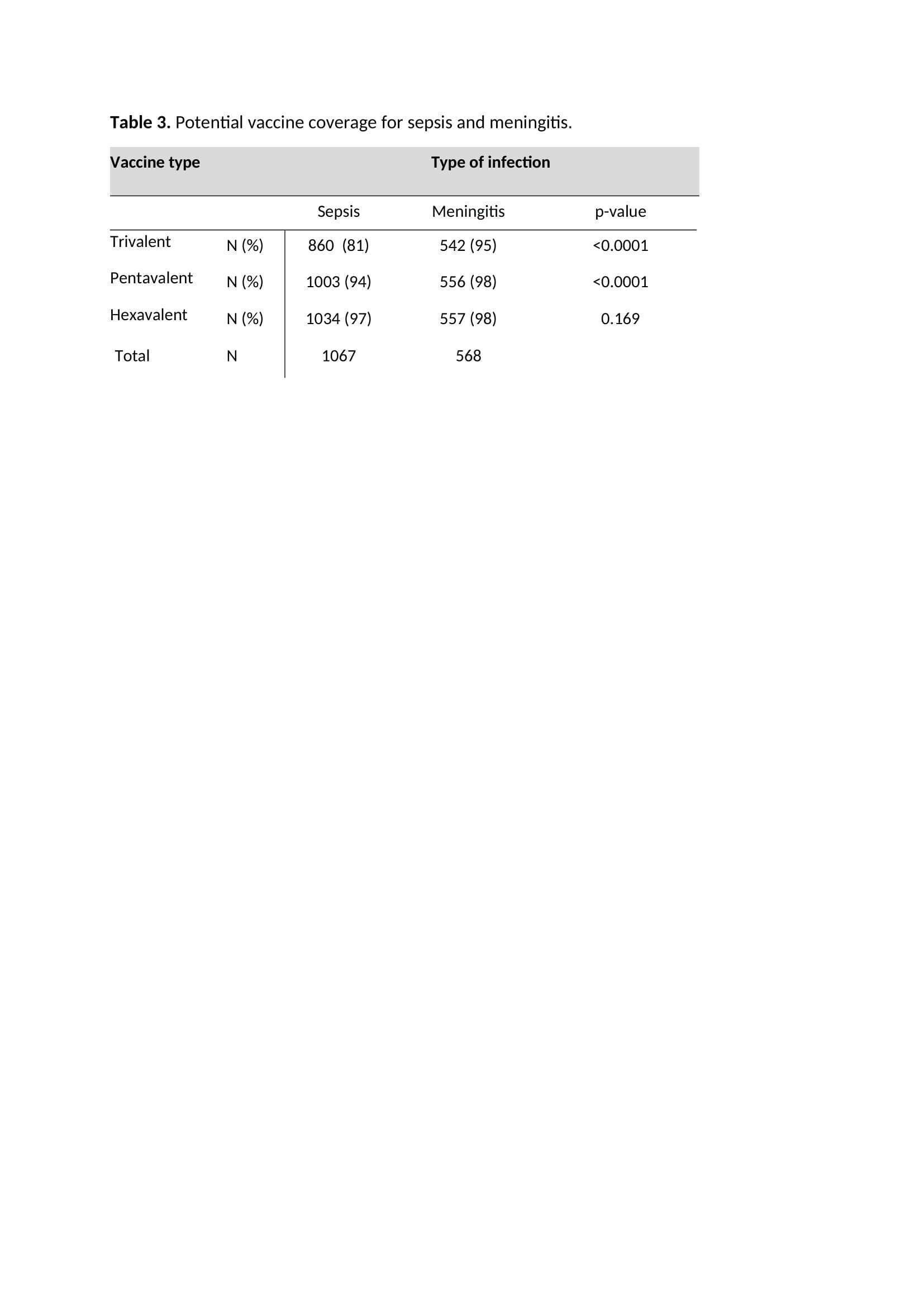
Results: A total of 1666 GBS episodes were identified. Serotype was available for 1635 (98%) episodes. Potential coverage was 86%, 95% and 97% for the tri-, penta- and hexavalent vaccine respectively (table 1). For the hexavalent vaccine, potential coverage increased significantly over time (table 2). For the tri- and pentavalent vaccine, potential coverage was lower in sepsis cases compared to meningitis cases (table 3).
Conclusion: A hexavalent vaccine would have covered nearly all GBS sepsis and meningitis cases in young infants in the Netherlands.
References
1. Absalon, J., et al., Safety and immunogenicity of a novel hexavalent group B streptococcus conjugate vaccine in healthy, non-pregnant adults: a phase 1/2, randomised, placebo-controlled, observer-blinded, dose-escalation trial. Lancet Infect Dis, 2020.
2. Bijlsma, M.W., et al., Epidemiology of invasive meningococcal disease in the Netherlands, 1960-2012: an analysis of national surveillance data. Lancet Infect Dis, 2014. 14(9).
Powered by Eventact EMS