
Establishing a Novel Model to Study Cardiac Electroporation Ablation Utilizing Human Induced Pluripotent Stem Cells
2Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel
3Talpiot Sheba Medical Leadership Program, Sheba Medical Center in Tel-Ha’shomer, Ramat-Gan, Israel
4Sohnis Family Laboratory for Cardiac Electrophysiology and Regenerative Medicine, Rappaport Faculty of Medicine, Technion – Israel Institute of Technology, Haifa, Israel
5Department of Cardiology, Rambam Health Care Campus; Haifa, Israel
Background: Cardiac electroporation is a promising novel non-thermal ablation method, gaining significant interest with recent first-in-man data suggesting effective cardiac lesion generation with no collateral damage. Nevertheless, significant knowledge gaps exist regarding the electrophysiological consequences of electroporation in cardiomyocytes, including; cell-specificity, protocol optimization, irreversibility-threshold, recovery time-constants, and the mechanistic nature of its cytolytic and anti-arrhythmic properties.
Purpose: Establishing an innovative in-vitro model for the study of cardiac electroporation-ablation, utilizing human induced pluripotent stem cells (hiPSCs).
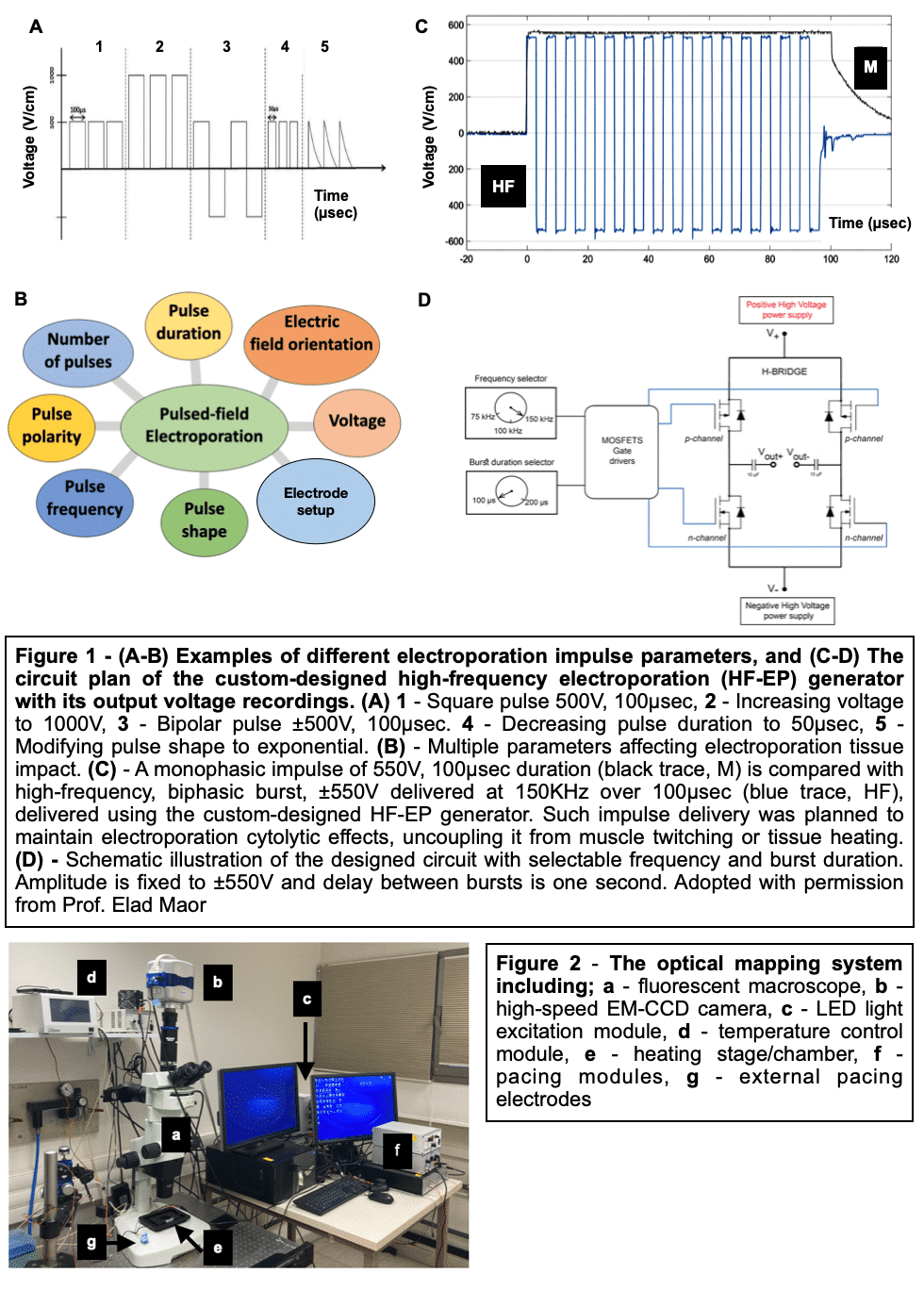
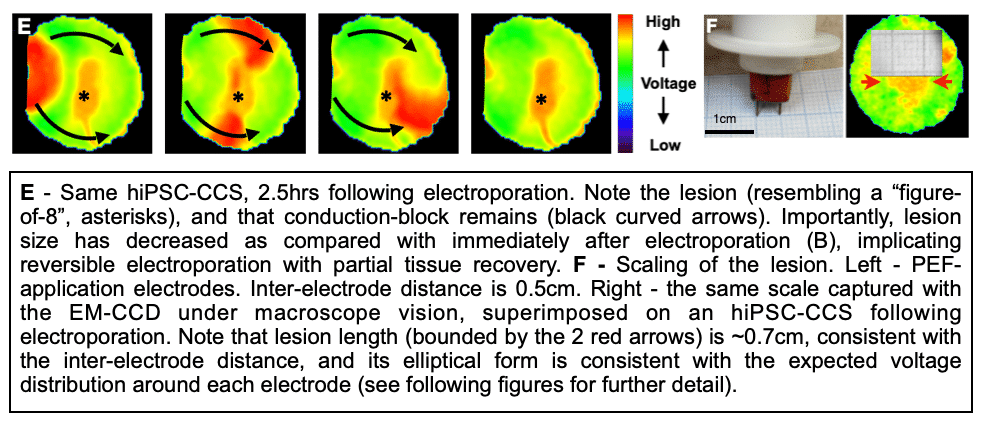
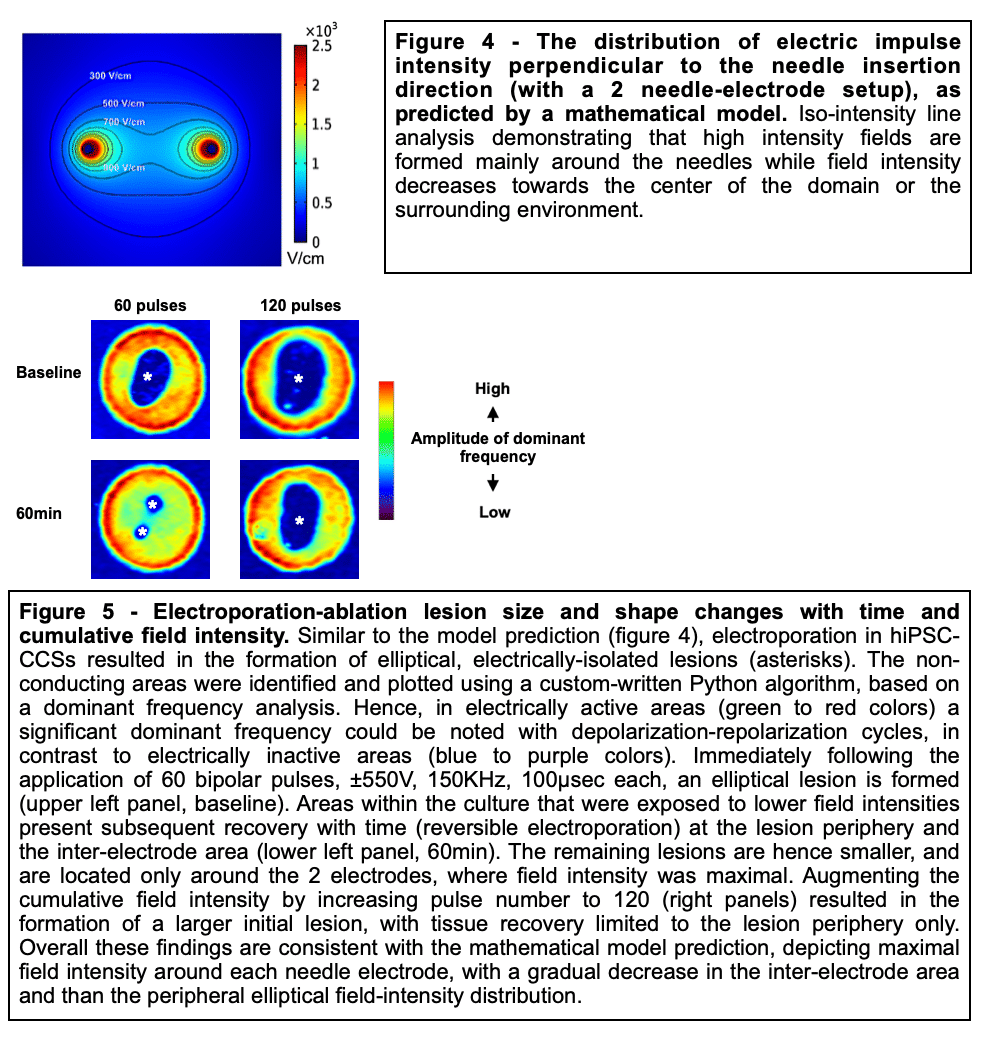
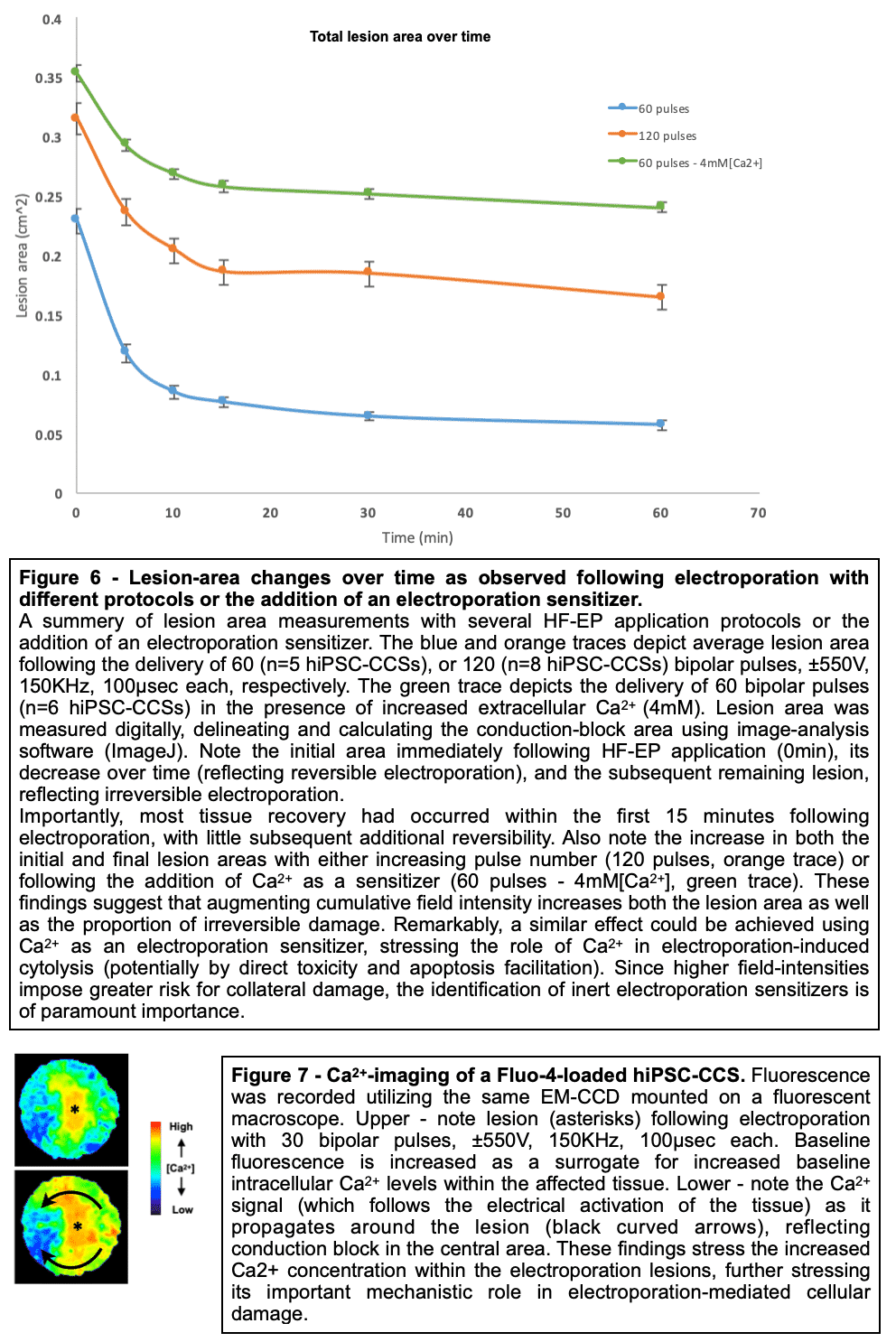
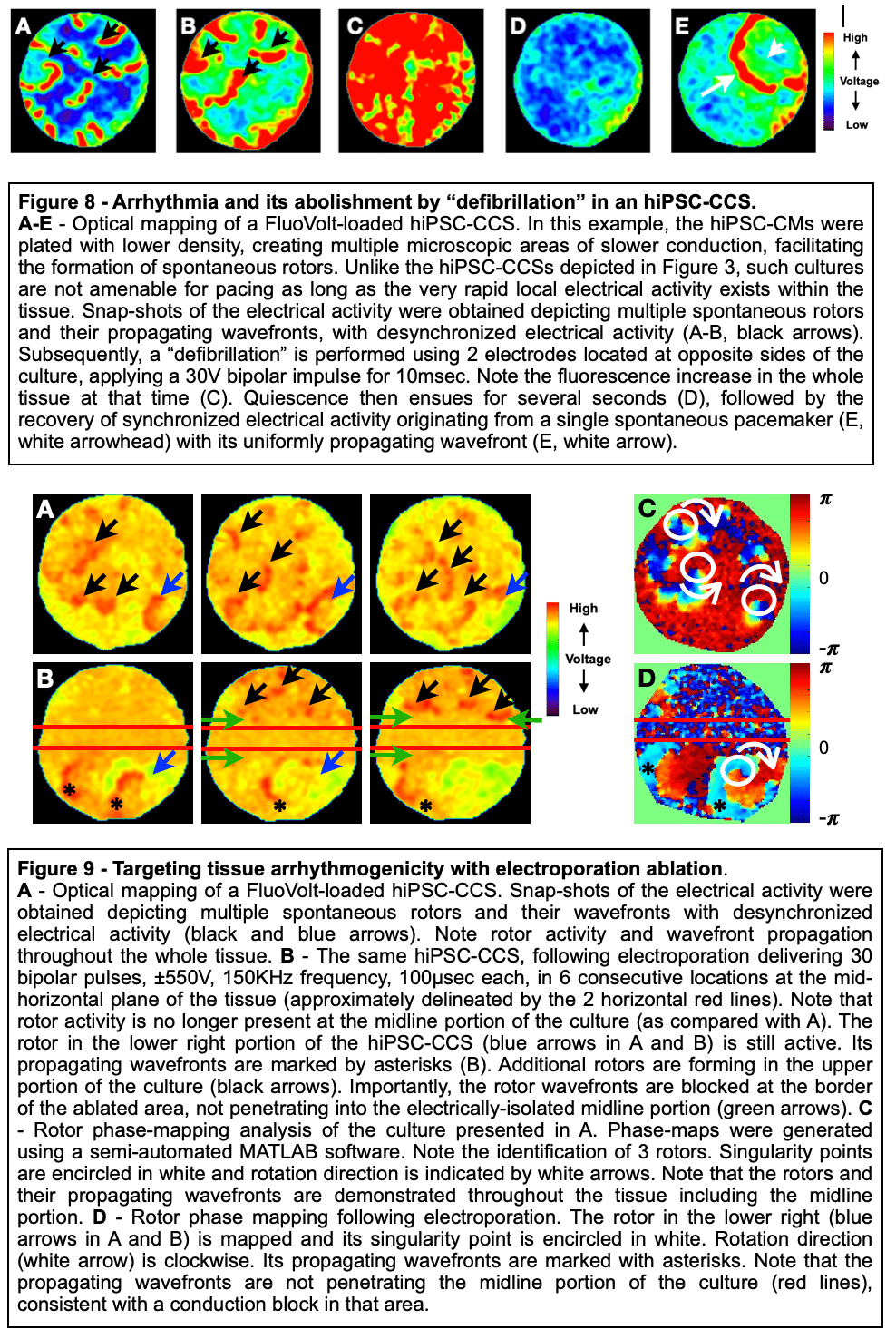
Methods and Results: Healthy-control hiPSC-derived cardiomyocytes were enzymatically dissociated and seeded as circular cell sheets (hiPSC-CCSs). Electroporation-ablation experiments were performed using a custom made high-frequency electroporation (HF-EP) generator. Two needle-shaped electrodes were used for HF-EP delivery. Subsequently, detailed voltage-mapping studies of the hiPSC-CCSs were conducted. HF-EP application generated electrically isolated lesions within the hiPSC-CCSs. Further characterization of the temporal changes and electrophysiological properties following electroporation revealed that; (1) lesions persisted over prolonged periods of time (days), indicating irreversible electroporation, (2) a temporal decrease in lesion dimensions was noted, consistent with a significant reversible electroporation component, (3) most tissue recovery occurred within the first 15 minutes following electroporation with little recovery beyond that time-frame, (4) increasing pulse-number augmented lesion area as well as the proportion of irreversible damage, and (5) electroporation sensitization was achieved by increasing extracellular Ca2+, indicating its crucial role in electroporation cytolysis, potentially via direct cellular toxicity and apoptosis facilitation. Finally, evaluating for HF-EP anti-arrhythmic properties, we targeted multiple rotors or focal triggered-activity generated in the hiPSC-CCSs. HF-EP application generated sustained line-blocks, isolating arrhythmogenic substrates within the hiPSC-CCSs.
Conclusion: Our results demonstrate the ability to study cardiac electroporation utilizing hiPSC-derived cardiomyocytes, provide novel insights into its temporal and electrophysiological characteristics, facilitate electroporation protocol optimization, screen for potential electroporation sensitizers, and to study its mechanistic nature and anti-arrhythmic properties.
Powered by Eventact EMS