
Whole anatomical rat atria superfusion as a model of atrial fibrillation pathogenesis and treatments
2Division of Cardiology, and Tamman Cardiovascular Research Institute, Leviev Heart Center, Sheba Medical Center - Tel Hashomer, Israel
3The Sackler School of Medicine, Tel-Aviv University, Israel
4Talpiot Sheba Medical Leadership Program, Sheba Medical Center - Tel Hashomer, Israel
5Cardiology Department, Rambam Health Care Campus, Israel
Introduction: Atrial fibrillation (AF) is the most common sustained clinical arrhythmia. One of the barriers to an improved mechanistic understanding of AF has been a lack of appropriate tissue models, especially in small animals. Here, we propose an advanced ex-vivo anatomical model, based on isolated rat atria, for acute assessment of AF susceptibility, atrial electrophysiological properties, drug testing and evaluation of new ablation modalities.
Experimental Methods: Wistar rats atria (N=25) were isolated, flattened and pinned to a custom-made silicon plate. Atria were superfused with an oxygenized Tyrode’s solution, loaded with a voltage-sensitive dye, and mapped using a high-resolution optical mapping system. AF was induced with 1uM carbamylcholine (N=23) coupled with pacing maneuvers. and treated with 30uM Vernakalant (N=10) or 10uM Flecainide (N=10). Finally, the feasibility of a new ablation technique (electroporation) was evaluated.
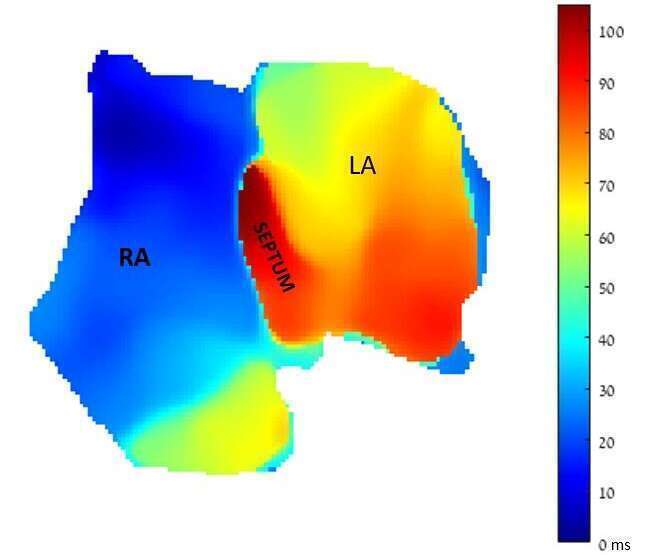
Results and discussion: Optical mapping results suggested that the superfusion procedure led to a fast atrial recovery. Sinus activity was conserved for all atria for a long period. All anatomical landmarks were clearly visualized. The acquired optical signals were analyzed during sinus rhythm and pacing, which allowed the creation of detailed activation maps and measurements of action potential duration (APD) and conduction velocity at different pacing rates. The resulting APD restitution curves revealed electrical excitation at high pacing rates with a relatively flattened curve. AF was induced and optical mapping confirmed the presence of reentrant activity. AF was successfully treated using Vernacalant and Flecainide. Finally, we demonstrated the feasibility of a new ablation approach (electroporation) for the creation of a continuous linear lesion serving as a functional block.
Conclusions: The isolated superfused atria model, coupled with optical mapping, can be utilized for long-term high-resolution functional imaging. This allows the study of the electrical activity during sinus rhythm and pacing activity, creation of arrhythmic activity, and assessment of different therapeutic measurements.
Powered by Eventact EMS