
Micro-engineered perfusable 3D-bioprinted glioblastoma model for in-vivo mimicry of tumor microenvironment
2Department of Pathology, Sackler Faculty of Medicine, Tel Aviv University, Israel
3Sagol School of Neurosciences, Tel Aviv University, Israel
Many new drugs show promising results in laboratory research, but eventually fail in clinical trials. The interactions of tumor and stromal cells in the microenvironment and the extracellular matrix are required to promote tumor progression and metastasis. Such interactions do not form in conventional 2D cancer cell cultures that grow on a rigid, plastic, petri dishes. Therefore, there is a need to develop a 3D model that better mimics the tumor microenvironment.
We created patient specific 3D-bioprinted vascularized and pefusable cancer models which recapitulate the complex tumor microenvironment, based on natural and synthetic polymers containing several types of cells, to serve as a novel platform for drug screening to determine the optimal personalized treatment.
We have synthesized and characterized a library of different polymeric hydrogels which can be used as bioinks that enable the formation of a printable soft-solid 3D structure in our self-designed bioreactor.
We have recapitulated the tumor heterogenic microenvironment in fibrin 3D-bioink by creating a penta-culture of glioblastoma tumor cells together with primary human or murine astrocytes, microglia, microvascular brain pericytes and endothelial cells. This 3D-bioprinted tumor resembles the tumor microenvironment using one polymer as bioink with high reproducibility and excellent uniformity. The second polymer is a sacrificial hydrogel which we used in order to create the perfusable blood vessels. We have observed similar growth curves, drug response and genetic signature of glioblastoma cells grown in our unique 3D-bioink platform and in in-vivo studies as opposed to those grown in 2D culture.
This 3D-bioprinted model could be the basis for potentially replacing cell cultures and animal models as a powerful platform for rapid, reproducible and robust personalized drug screening, drug development and target discovery.
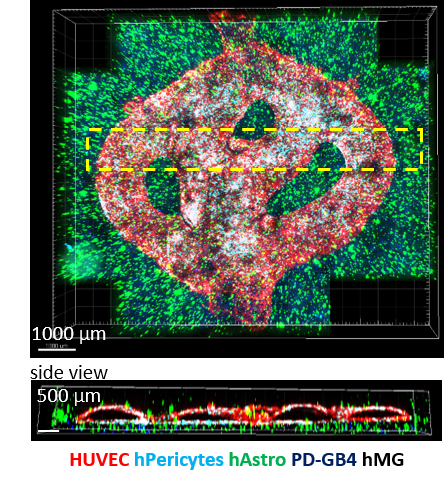
Tiled Z-stack confocal microscopy images of the 3D-bioprinted penta-culture vascularized glioblastoma model.
Powered by Eventact EMS