
Antibody guided, activatable NIR photosensitizer for targeted photodynamic cancer therapy with reduced side-effects
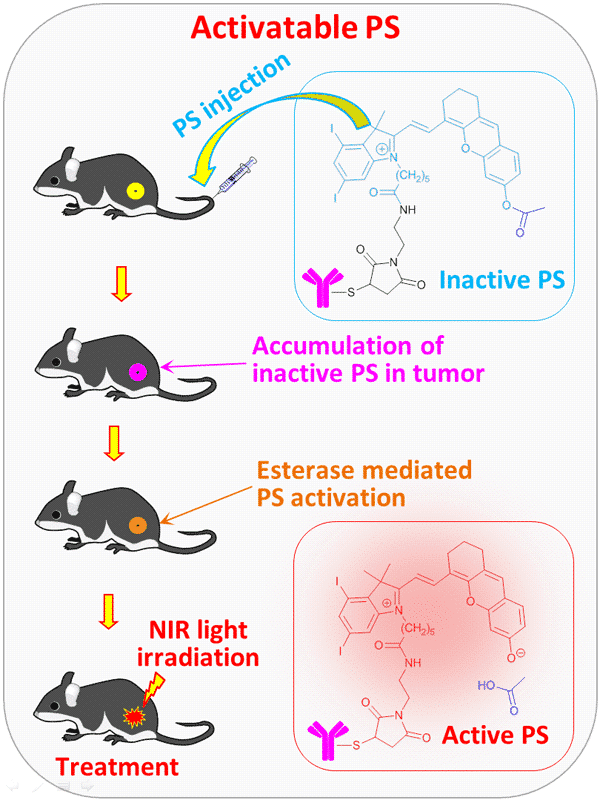
Photodynamic therapy (PDT) is a well-known, powerful, and relatively safe method for cancer treatment. PDT utilizes a long-wavelength (near-IR) absorbing dye termed as photosensitizer (PS) to kill cells under light exposure. A known shortcoming of existing photosensitizers is that they are constantly active and, therefore, may cause toxic side effects to organs upon occasional light exposure during transportation with the bloodstream. To overcome this issue, we developed an activatable, antibody-guided photosensitizer Ab-mI2XCy-Ac, where the inactive dye mI2XCy-Ac, deactivated by the acetyl (Ac) protected hydroxyl group, was linked to a targeting monoclonal antibody (Ab) Trastuzumab (Herceptin) specific to Her2 receptors. This inactive photosensitizer was shown to be safely, without detectable side-effects delivered to targeted tumor, where it is activated by the esterase-mediated acetyl group cleavage and effectively treat the tumor upon the near-IR light-irradiation. The PDT efficacy and side effects were investigated in the mice model with Her2 positive human breast cancer cell line BT-474 as compared to constantly active photosensitizer Ab-mI2XCy. The PDT efficacy of this novel photosensitizer, Ab-mI2XCy-Ac, was shown to be the same as for the constantly active Ab-mI2XCy, while the side effects are noticeably reduced. In addition, the activatable photosensitizer enables fluorescence monitoring of the activation events in the body. This new photosensitizer demonstrates a novel approach for designing novel, highly efficient photosensitizing systems with reduced side effects.
Powered by Eventact EMS