
Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases
2The Norman Seiden Multidisciplinary Program for Nanoscience and Nanotechnology, Technion – Israel Institute of Technology, Israel
3Department of Biochemistry and Molecular Biology, Faculty of Science and Technology, University of the Basque Country (UPV/EHU), Spain
4Department of Developmental Biology and Cancer Research, The Institute for Medical Research Israel-Canada, The Hebrew University-Hadassah Medical School, Israel
5Clinical Neurosciences Research Laboratory, Health Research Institute of Santiago de Compostela (IDIS), Spain
6Life Sciences and Engineering Infrastructure Center, Lorry I. Lokey Interdisciplinary Center, Technion – Israel Institute of Technology, Israel
7Pathology Institute, Tel Aviv Sourasky Medical Center, Israel
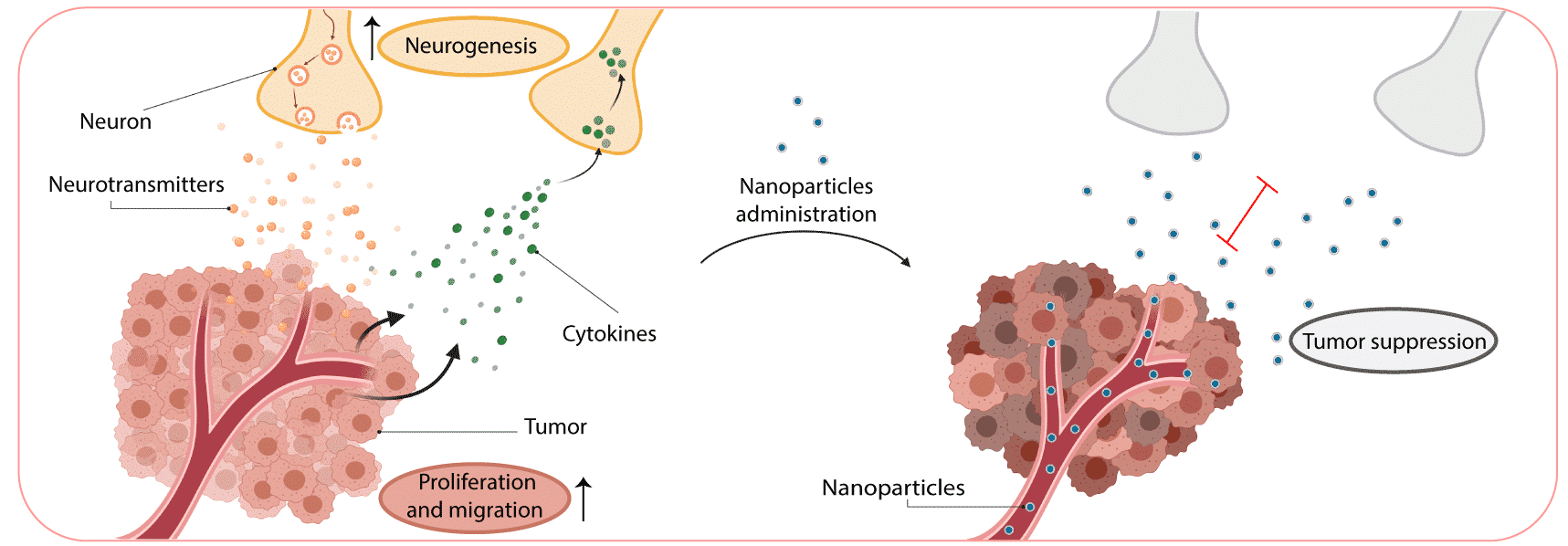
Neurons within the tumor microenvironment promote cancer progression. Cancer cells can grow and invade the nerves in the tumor microenvironment, and use it as a mean for metastatic spread. Moreover, nerves and their axons infiltrate the tumor tissue and stimulate cancer-cell growth, proliferation, and invasion. We studied the nerve/cancer cross-talk and how nanotechnology can be utilized to target breast-cancer neurons, modulate and interfere their signaling, in order to inhibit cancer development. Bupivacaine is a non-opioid sodium channel blocker that selectively interrupts the transmission of nerve impulse. Therefore, bupivacaine can be used as a molecule that targets neurons within the tumor microenvironment, while also potentially relieving cancer-associated pain. Systemic administration of free bupivacaine is associated with cardiovascular and central nervous system toxicity. In our study, bupivacaine loaded liposomes were developed to curb tumor progression by targeting the neurons within breast cancer tumors. We detected neuron cells and fibers within triple-negative breast cancer (4T1) sections, highlighting the potential of nerve involvement in tumor progression in vivo. The co-culture of neuron cells with cancer cells stimulated cancer cell migration and proliferation. However, the addition of bupivacaine, reduced neurons viability and signaling, followed by a decrease in cancer cells proliferation. When the liposomes were intravenously injected into mice bearing orthotopic 4T1 tumors, a favorable accumulation of liposomes in the tumor and their distribution with the tumor neurons was observed. Moreover, tumor growth and metastasis formation was reduced when liposomal bupivacaine was intravenously injected. Overall, this study presents a potentially novel clinical strategy for cancer therapy using non-opioid anesthetic nanoparticles to target nerves in breast cancer tumor.
(1) A. H. Zahalka, P. S. Frenette, Nerves in cancer. Nature Reviews Cancer 20, 143-157 (2020).
(2) P. Jobling, J. Pundavela, S. M. Oliveira, S. Roselli, M. M. Walker, H. Hondermarck, Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res 75, 1777-1781 (2015).
Powered by Eventact EMS