
Modified halloysite nanotubes for targeting bacterial cells
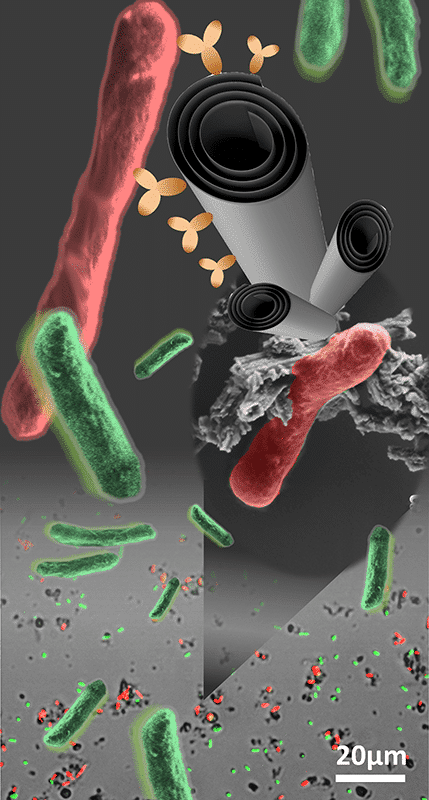
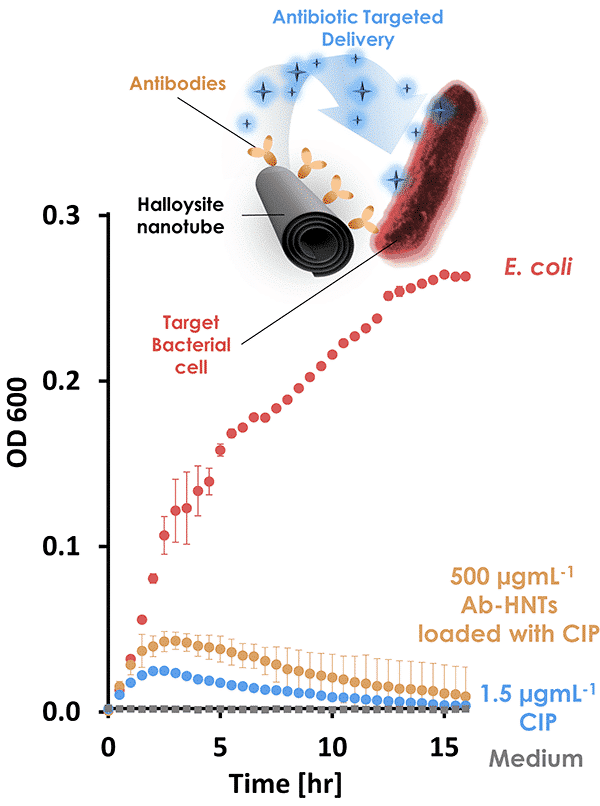
Imbalanced microflora, or dysbiosis, is an unhealthy condition that could be induced by non-specific antibiotic treatment. One approach to overcome this challenge is the targeted delivery of antibiotics by nanocarriers. In this work, we demonstrate for the first time that clay Halloysite nanotubes (HNTs) can be tailored to selectively bind with specific bacterial cells and deliver an antimicrobial payload, see Figures. HNTs are abundant intrinsically mesoporous nanotubes that are ideal for the loading and sustained release of bioactive compounds. In addition, HNTs are known to interact with biological cells in various ways, such as adsorption to bacterial cells and incorporation into the bacterial biofilm(1). To endow HNTs with selective binding capabilities, HNTs surface is first functionalized with a capture probe (immobilized antibodies). Then, the modified HNTs are loaded with a potent antibiotic (ciprofloxacin) to be gradually released by diffusion only at the proximity of target bacteria for specific neutralization. The chemical modification of HNTs surface begins with an acidic etching to expose additional reactive hydroxyl groups and increase the specific surface area (up to ca. 130 m2g-1). Then, the etched clay nanotubes are amino-silanized and carboxylated towards a rapid conjugation of protein A onto HNTs surface. This protein acts as an anchorage site for IgG immobilization at a proper orientation to maximize antibody activity. Physiochemical characterization confirms the immobilization of IgG onto HNTs surface. The realization of selective binding capabilities is investigated both qualitatively and quantitatively by fluorescence microscopy and high-throughput imaging flow cytometry, respectively. Spectroscopic analysis of ciprofloxacin indicates the successful loading onto HNTs by adsorption coupled with a sustained release profile. The released antibiotic payload retains its antibacterial activity against target bacteria as studied by the plate count and microbroth dilution methods. The suggested HNT-based smart carrier constitutes a generic platform for targeted delivery that could be selectively tailored against any microorganism by facile antibody adjustment.
Reference 1) Prinz Setter, O. & Segal, E. Nanoscale 12 (2020).
Powered by Eventact EMS