
Delivery of iodinated heptamethine indocyanine photosensitizing
drugs to gram-positive and gram-negative pathogens for
antimicrobial photodynamic therapy (APDT)
2Department of Luminescent Materials and Dyes, SSI “Institute for Single Crystals” of the NAS of Ukraine, Ukraine
3Department of Chemical Engineering, Ariel University, Israel
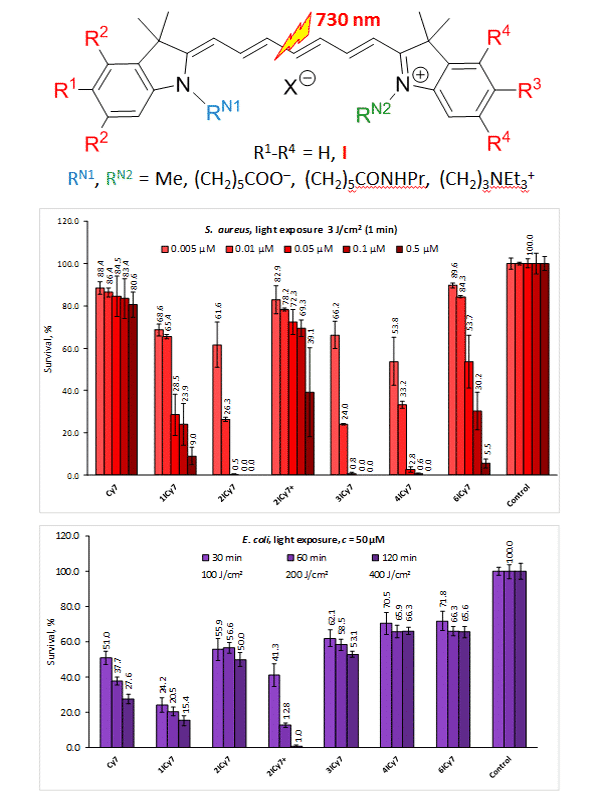
We developed a series of heptamethine indocyanine dyes containing different solubilizing groups (carboxy, triethylammonium, and amide) and a variable number (up to six) of iodine atoms facilitating generation of cytotoxic species upon photodynamic (PDT) and antimicrobial photodynamic (APDT) treatment. It has been proven that these dyes can perform as photosensitizing drugs efficiently killing Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) microbial pathogens when exposed to near-IR light. The effect of the solubilizing groups and the number of iodine atoms on photosensitizer uptake by these bacteria and photokilling ability was investigated.
In general, the introduction of heavy atoms such as iodine into organic dye molecules improves phototoxicity, which is attributed to the increasing probability of spin-orbit coupling resulting in the elevated rates of reactive species generation. We found, however, that the increasing number of iodines can decrease photokilling effect due to the reducing dye uptake. Thus, the efficacy of S. aureus photokilling increases with increasing the number of iodine atoms up to two, remains almost unchanged for the two-, three- and four-iodinated dyes, and reduces in the case of the hexa-iodinated cyanine. The mono-iodinated dye exhibits the most pronounced phototoxic effect to E. coli and P. aeruginosa. A positive charge provided by a triethylammonium group decreases photokilling of S. aureus but improves inactivation of E. coli and P. aeruginosa. Negatively charged carboxylic group noticeably decreases phototoxicity while the amide group recovers photokilling efficacy. Importantly, the carboxylic function enables linkage of the developed photosensitizers with bacteria-specific carriers such as antibody to yield targeting phototoxic drug delivery systems.
Powered by Eventact EMS