
Studying the reactivity of micellar nanoreactors by adjusting the hydrophobicity of the amphiphile and the substrate for palladium mediated depropargylation reaction in aqueous media
2Tel-Aviv University Center for Nanoscience and Nanotechnology, Tel Aviv University, Israel
3Adama Center for Novel Delivery Systems in Crop Protection, Tel Aviv University, Israel
4Blavatnik Center for Drug Discovery, Tel Aviv University, Israel
5The Center for Physics and Chemistry of Living Systems, Tel Aviv University, Israel
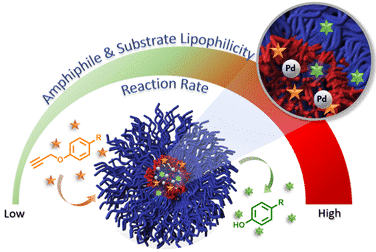
Bioorthogonal chemistry is a powerful tool for therapeutic and imaging applications as it allows in-vivo transformations of molecules, which are not possible through naturally occurring bioprocesses. Since many of bioorthogonal reactions are based on transition metal chemistries, translating them to an aqueous setup is major challenge as the surrounding water molecules could poison the catalyst in addition to issues of solubilizing the reagents. Polymeric self-assemblies, such as micelles, can act as nanometer-sized flasks to dissolve and provide the necessary lipophilic environment for conducting these reactions.1 Considering the huge potential of such systems, we wanted to systematically elucidate the effect of hydrophobicity of both, the amphiphile and the substrate on the reaction kinetics in an aqueous environment. Towards this goal, we designed a set of palladium-embedded micelles, made from PEG-dendron amphiphiles and studied their ability to facilitate palladium mediated depropargylation reactions2 of four substrates with increasing hydrophobicities (logP). Using dendrons to form hydrophobic block allowed us to fine-tune the lipophilicity of the dendritic end-groups and study how precise structural changes in the hydrophobicity of the amphiphiles affect the reaction rates. The kinetic data revealed linear dependence between the rate constants and the hydrophobicity of the amphiphiles, while exponential dependence was observed for the lipophilicity of the substrates . Our observations show the important roles that the hydrophobicity of both the substrates and the amphiphiles play in the lipo-selectivity of nanoreactors, illustrating the possibility of tweaking hydrophobicity as a tool for optimizing the reactivity and selectivity of nanoreactors.3
References:
(1) Lipshutz, B. H. The Nano-to-Nano Effect Applied to Organic Synthesis in Water. Johnson Matthey Technol. Rev. 2017, 61, 196–202.
(2) Pal, M.; Parasuraman, K.; Yeleswarapu, K. R. Palladium-Catalyzed Cleavage of O/N-Propargyl Protecting Groups in Aqueous Media under a Copper-Free Condition1. Org. Lett. 2003, 5 (3), 349–352.
(3) Tevet, S.; Wagle, S. S.; Slor, G.; Amir, R. Tuning Reactivity of Micellar Nanoreactors by Precise Adjustments of the Amphiphiles and Substrates Hydrophobicity. 2021, 1–12.
Powered by Eventact EMS