
Image Guided Targeted Delivery of Trastuzumab Conjugated Cy5 and SN-38 in Breast Cancer
2Department of Molecular Biology, Ariel University, Israel, Israel
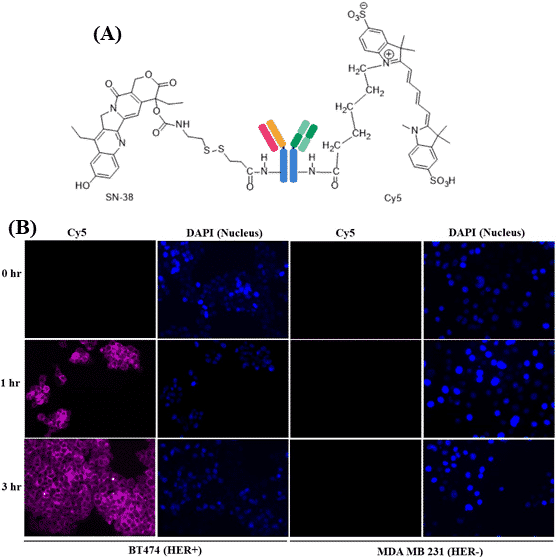
According to World Health Organization, cancer is one of the leading causes of death worldwide, and nearly 10 million people died in the year 2020. One of the major drawbacks of the current modalities of the treatments is the minimal availability of the drug to the tumor site and the accumulation of drug at a non-target site that leads to systemic toxicity as a side effect of the anti-cancer drug. Another problem is the inability to monitor the accumulation/distribution of the drug during the treatment. To circumvent these, we have designed an SN-38 and Cy5 loaded trastuzumab (Herceptin) for the image-guided delivery of the SN-38 to the tumor. The SN-38 and Cy5 were conjugated to the antibody with the drug to protein ratio of 3.7 and 4.3 respectively. In-vitro internalization study with HER+ and HER- cells demonstrated the specificity of developed antibody-drug conjugates. The antibody-drug conjugates significantly increased the internalization of Cy5 in HER+ positive BT474 cells whereas no traces of Cy5 was found inside the HER- MDA MB 231 cells under the same treatment conditions. We will be further evaluating the in vitro cytotoxicity, apoptosis assay of free SN-38 versus antibody-drug conjugates in series of both HER+ and HER- cells. Next, the conjugates will be evaluated for anti-tumor efficacy and in vivo imaging of tumors in mice using a live cell imager. The antibody simultaneously tagged with anticancer drug and dye promises a novel theragnostic modality for the image-guided targeted delivery of the drug to the tumor.
References
1. Vasir, J. K., & Labhasetwar, V. (2005). Targeted drug delivery in cancer therapy. Technology in Cancer Research and Treatment, 4(4), 363–374.
Powered by Eventact EMS