
Targeting relaxin receptor as a therapeutic approach for ovarian cancer
2Department of Materials Sciences and Engineering, Tel Aviv University, Israel
3Center for Nanoscience and Nanotechnology, Tel Aviv University, Israel
4Cancer Biology Research Center, Tel Aviv University, Israel
Epithelial ovarian cancer is the leading cause of death from gynecological cancers in the developed world. High-grade serous ovarian cancer (HGSOC) is the most lethal subtype of the disease, accounting for more than 70% of ovarian cancer associated mortalities. Despite the initial efficacy of surgical de-bulking combined with chemotherapy, more than three-quarters of SOC patients relapse and die from the disease. There is, therefore, an urgent need to develop more effective ways to treat HGSOC.
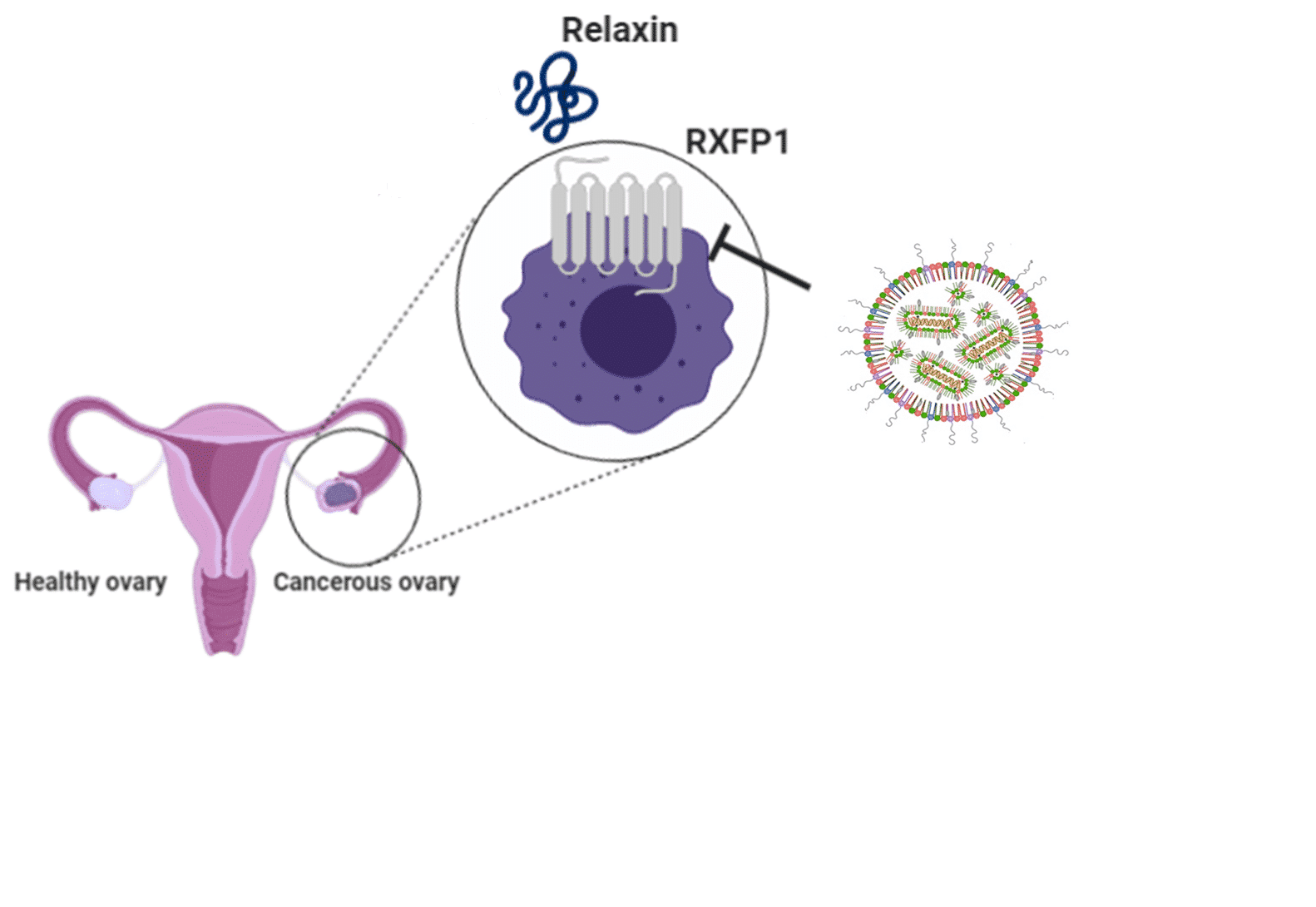
The Relaxin receptor (RXFP1) have been previously reported to promote cell proliferation and increase invasiveness and migration of breast, endometrial, thyroid and prostate cancer cells. In this study, we aimed to evaluate RXFP1 as a therapeutic target in ovarian cancer, by nucleic acid drug delivery.
siRNA- mediated knock down of RXFP1 in ovarian cancer cell lines resulted in decreased cell proliferation, migration and cell cycle disruption, validating RXFP1 as a therapeutic target in this indication. For in vivo delivery, ionizable lipid-based nanoparticles (LNPs( entrapping RXFP1 siRNA were used as a safe, non-immunogenic delivery method. When targeted by antibodies, siRNA carriers could be expected to accumulate in a specific tissue or cell type, releasing their payload only at the desired location and enable a precise therapeutic effect. We developed targeted LNPs utilizing our recently designed modular targeting platform, named ASSET.
Here, the therapeutic potential of antibody-targeted LNPs entrapping RXFP1 siRNA was evaluated using a human ovarian cancer xenograft mouse model. In a bio distribution study, we show that intraperitoneally administered LNPs targeted by an anti-human epidermal growth factor receptor (hEGFR) antibody tend to accumulate favorably in the tumors surrounding the ovaries. Using LNPs encapsulating siRNA to RXFP1, significant silencing of the receptor in the tumors was achieved, leading to reduced tumor burden and increased survival of the mice. Taken together, our data validates the tumor promoting properties of the relaxin signaling axis, and demonstrates the feasibility of targeting RXFP1 as a therapeutic approach for ovarian cancer.
Powered by Eventact EMS