Immunotherapy Induced Glycemic Dysregulation – Characterization and Proposed Mechanisms Beyond Beta-Bell Autoimmunity
2Sackler Faculty of Medicine, Tel Aviv University
3Gertner Institute for Epidemiology and Health Policy Research,, Sheba Medical Center
Aim:
Immunotherapy has revolutionized cancer treatment while introducing a new spectrum of immune-related adverse effects (irAE). Hyperglycemia is frequently observed after initiation of immunotherapy. Immune-mediated type 1 diabetes mellitus, currently the only defined condition, represents only a minority of cases (estimated prevalence 0.5%). We aimed to characterize dynamics and effectors of glycemic dysregulation following immunotherapy.
Methods: A retrospective study, using the MD-Clone interface to retrieve electronic medical data of all cancer patients treated with immunotherapy during the years 2015-2021.
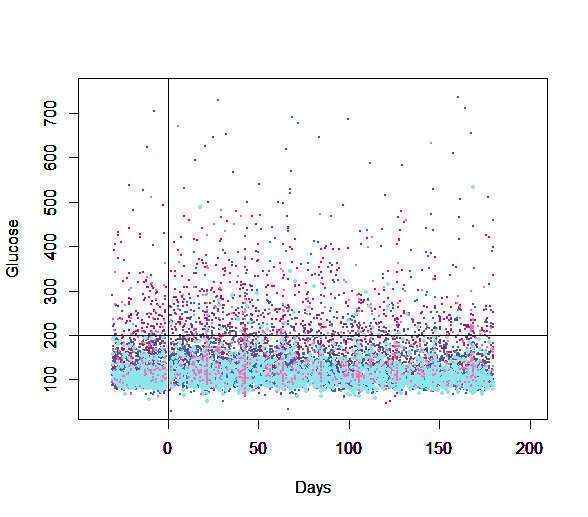
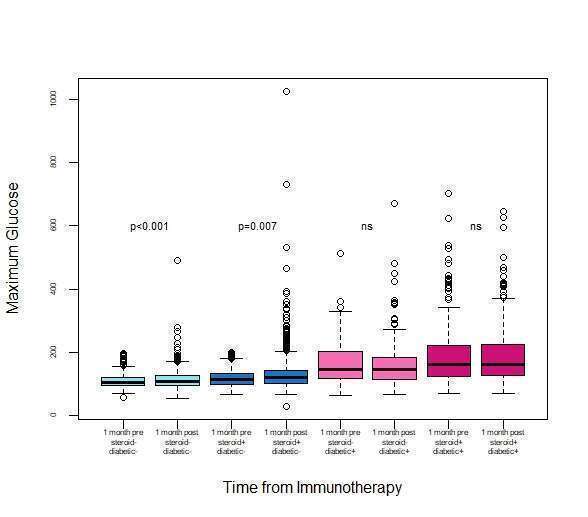
Results: Among 3384 patients, a statistically significant increase in glucose levels was observed after initiation of immunotherapy [mean of maximum 132.3mg/dl ± 55.8 vs 139.8 ± 64.6 (p-value<0.001)]. Glycemic dysregulation was significant in non-diabetic patients [n=2549, mean of maximum 109.6 mg/dl ±22.9 vs 114.3 mg/dl ±31.4, p<0.001], and was aggravated by glucocorticoids (anti-emesis for chemo-immunotherapy protocols or for irAEs [118mg/dl ±26.2 vs 130.6 mg/dl ±53.2 p<0.007]. Diabetic patients, though, presenting with significantly higher glucose levels and wider variability, both aggravated by glucocorticoid therapy, had no significant increase in glucose levels after initiating immunotherapy.
Immunotherapy induces a significant glycemic dysregulation among non-diabetic cancer patients, assumingly via an increase in insulin resistance resulting from cytokine flare and systemic inflammation. This effect is assumingly concealed among diabetic patients, in whom multiple baseline distorted mechanisms are involved in glycemic dysregulation. The possibility of a distinct, reversible, autoimmune damage to beta cells would require evaluation in a prospective study with a dynamic assessment of the beta-cell reserve.