
ENGINEERING OF FUNCTIONAL SUPER-MOLECULAR STRUCTURES
2Biotechnology and Food Engineering, Technion - Israel Institute of Technology, Haifa, Haifa, Israel
In nature, numerous of proteins self-assemble into Super-Molecular Structures (SMSs) (1-2). Recent studies describe engineering of diverse functional materials, based on SMS, into smart materials for different purposes, e.g., tissue scaffolds, biomedical devices coatings, antiseptic materials and more (3-4). Due to limitation of high resolution of the structure of SMSs, deep understanding of SMSs based materials is limited. Herein, I present an approach to better understand and engineer protein based functional SMSs. In four sequential works, we rely on a high resolution atomic structure combined with electron and light microscopies to investigate the estate of the SMSs, their surface morphology, complexation and localization. This, we believe, can greatly promote our ability to interpret SMSs, and to further develop and engineer smart materials based on SMSs.
Initially, we determined the atomic structure of a SMS formed by a human derived antimicrobial peptide. Antimicrobial peptides (AMPs) are a diverse group of biological antibiotic compounds found in all kingdoms of life, which can form SMSs (4-6). Using European synchrotron beam radiation source, we determined the crystal structure of a human derived AMP, LL3717-29, in its SMS form in a very high resolution (1.35 Å). The unique architecture of the fibrillar assembly is seen in Figure 1; SMS of chains of amphipathic helices that exposes mixed (hydrophobic-cationic) interfaces. We correlated between the self-assembly, the atomic structure, and the thermal stability and antibiotic activity of LL3717-29 (5).
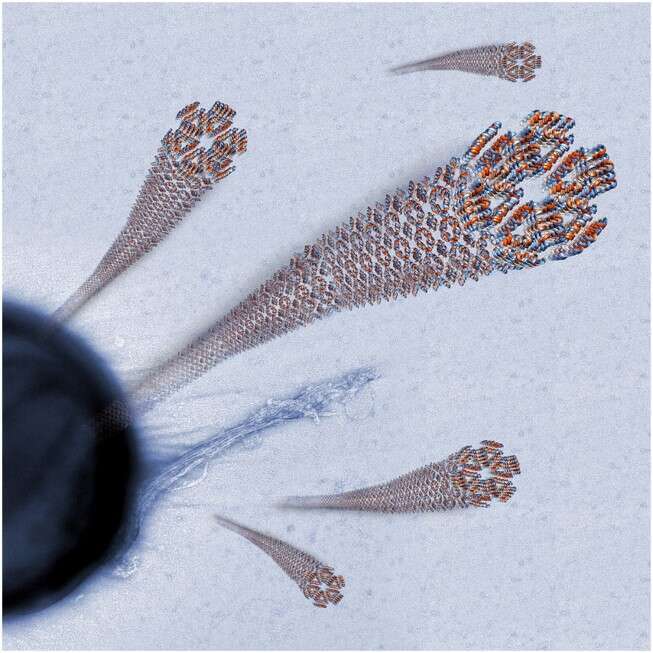
Figure 1. Atomic structure and fibril of LL3717-29 in its SMS form. An electron micrograph image presenting the fibril merging out from a bacteria membrane. The atomic structure of LL3717-29 is emphasizing the SMS architecture, annotated by colors: red= hydrophobicity, blue = cationic charge.
Second, deciphering LL3717-29’s atomic structure and its chemical-physical properties (5), we have engineered a molecular switch which created controllable smart antibiotic particles (Figure 2. A). Tunable compounds can be applied in many therapeutic and biomedical application to overcome resistance. The switch enabled us to alternate the peptides assembly into different SMSs, toward active (“on”) and non-active (“off”) species (Figure 2. A). The alternations between the two states of activity can be done easily and efficiently. Ultimately, these findings were demonstrated as a platform that could be applied on many AMPs to create a controllable antibiotic compound (recently published (6)).
Third, we harnessed the chemical properties of LL3717-29 to create conductive wires made of SMSs, for astronauts’ spacesuits textile. Space missions carrying high risk of Differential Charging (DC); an electrical phenomenon that threatens the life of astronauts in space (7-8). Conductive elements are essential components in space suites textile to reduce the risk to provoke DC (9). This application is based on the conjugation of lipid moieties to chains of LL3717-29 that self-assemble into cationic surfaced bundles (Figure 2. B, Engelberg et. Al, in preparation). This work was done with a collaboration with Prof. Nurit Ashkenazi, from the Material Sciences Dept. at the Ben Gurion University (BGU). My part of this work is to design and assemble the SMSs, and to characterize them in terms of visualization, biophysical assays, stability and size, biological functions (toxicity and antimicrobial activity) etc.
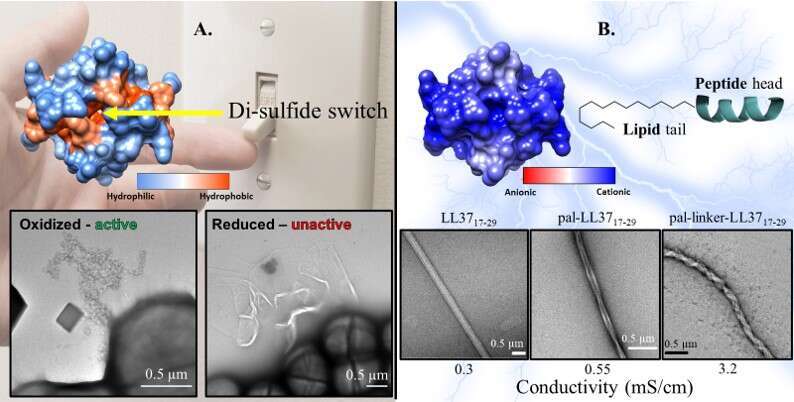
Figure 2. Engineering of functional SMSs based on LL3717-29. A. A molecular switch was designed to form covalent bonds in the center of the hydrophobic core. The addition of reducing factors alternates the SMSs formed into a non-active structure. B. According to the bold cationic surface charge (colored in blue), lipids conjugated to the peptides pushed the lipo-peptide into a SMS with improved conductivity.
Last but not least (fourth), to further explore our ability to design SMSs, we expanded our studies to food chemistry: We employed the atomic a popular milk protein, α-lactalbumin (ALA,(10)), to engineer fibrils SMSs with enhanced stability and which is capable of encapsulating lipophilic compounds. We observed a dramatic increasing of the bio-accessibility of capsaicin (CAP), a health beneficial compound originated from hot peppers (11): This due to its encapsulation within fibrils of ALA, protecting him from the harsh proteolytic environment in the gut, allowing it to gradually release CAP in the intestine (Figure 3, Food Hydrocolloids, 2022, In review). This work was done in collaboration with Prof. Uri Lesmes from The Biotechnology and Food Engineering (BFE) Dept. at The Technion. My role in this work was to design and assemble the supramolecular structures of ALA, to characterize their aggregation properties, to monitor them by imaging (TEM and confocal microscopy) and to perform bio-physical assays.
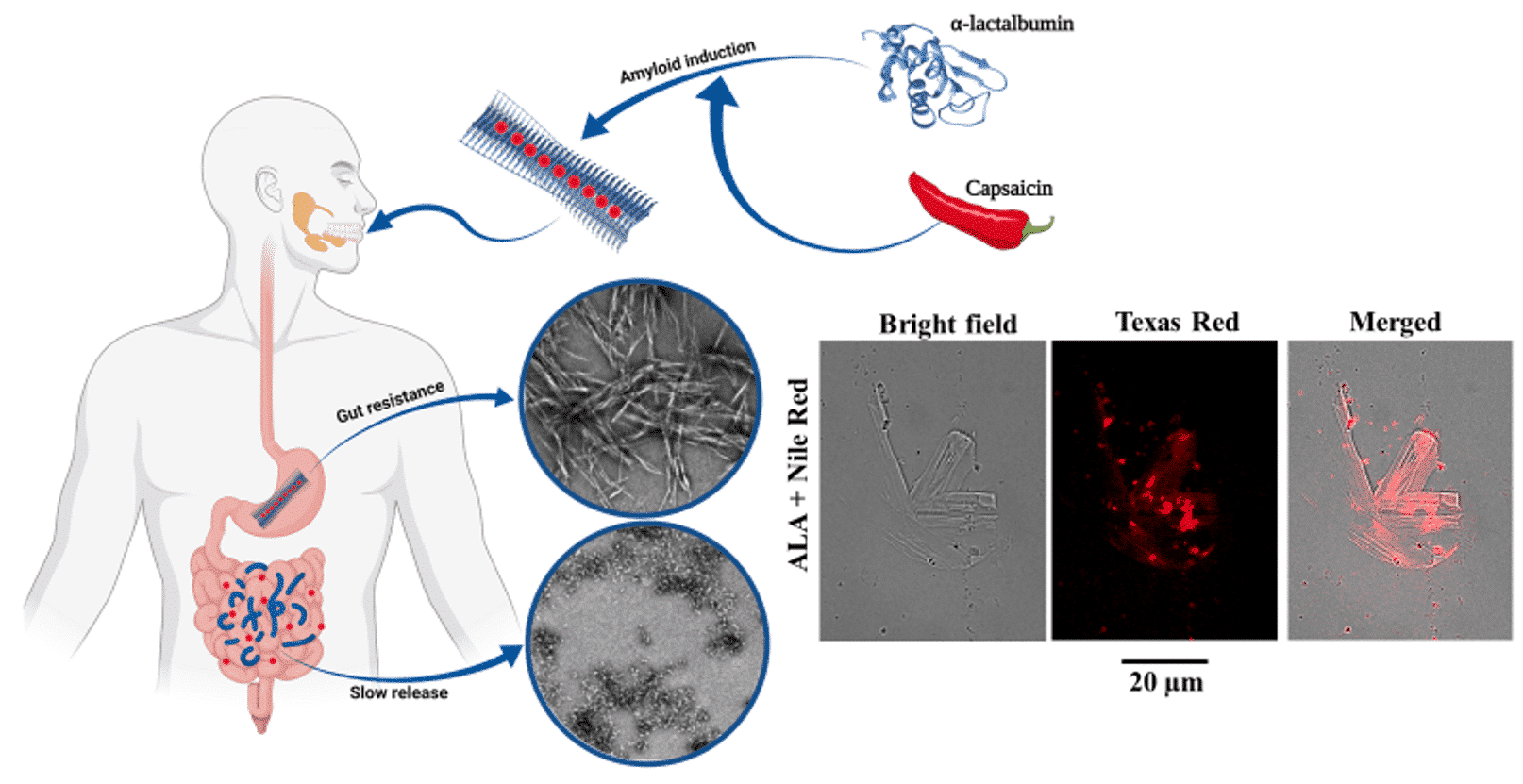
Figure 3. ALA based fibrils with gastric delivery capabilities. The scheme presenting assembly of ALA (blue) into SMS, and CAP (red) encapsulated within its fibrils. The SMS-CAP complexes survive the guts, and release CAP in the intestine. Electron and light microscopy (encapsulating NileRed, lipophilic fluorophore) are being used to monitor the survival of the fibrils along the gastric process.
Overall, this proposal emphasizes that high-resolution atomic information and surface imaging methodologies are powerful techniques to study and engineer functional SMSs. Hence, this approach can greatly advance design of many applications, in many different fields of science and engineering.
References:
1 van der Linden, E. & Venema, P. Self-assembly and aggregation of proteins. Current Opinion in Colloid & Interface Science 12, 158-165 (2007).
2 Ringler, P. & Schulz, G. E. Self-assembly of proteins into designed networks. Science 302, 106-109 (2003).
3 Knowles, T. P., Oppenheim, T. W., Buell, A. K., Chirgadze, D. Y. & Welland, M. E. Nanostructured films from hierarchical self-assembly of amyloidogenic proteins. Nature nanotechnology 5, 204-207 (2010).
4 Bai, Y., Luo, Q. & Liu, J. Protein self-assembly via supramolecular strategies. Chem Soc Rev 45, 2756-2767, doi:10.1039/c6cs00004e (2016).
5 Engelberg, Y. & Landau, M. The Human LL-37 (17-29) antimicrobial peptide reveals a functional supramolecular structure. Nature communications 11, 1-10 (2020).
6 Engelberg, Y., Ragonis-Bachar, P. & Landau, M. Rare by Natural Selection: Disulfide-Bonded Supramolecular Antimicrobial Peptides. Biomacromolecules (2022).
7 Katz, I., MANDELL, M., JONGEWARD, G., LILLEY, J., J & HALL, W. in Shuttle Environment and Operations II Conference. 7035.
8 Chou, K., Wang, A., Yu, W. & Wang, J. Laboratory experiments on dusty spacesuit charging and arcing in plasma. IEEE Transactions on Plasma Science 47, 3898-3904 (2019).
9 Barr, T. L. Studies in differential charging. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films 7, 1677-1683 (1989).
10 Chrysina, E. D., Brew, K. & Acharya, K. R. Crystal structures of apo-and holo-bovine α-lactalbumin at 2.2-Å resolution reveal an effect of calcium on inter-lobe interactions. Journal of Biological Chemistry 275, 37021-37029 (2000).
11 McCarty, M. F., DiNicolantonio, J. J. & O`keefe, J. H. Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart 2, e000262 (2015).