Invited: SPECTROSCOPY AND MICROSCOPY OF CATALYSTS AT WORK FOR ENERGY TRANSITION-RELATED PROCESSES
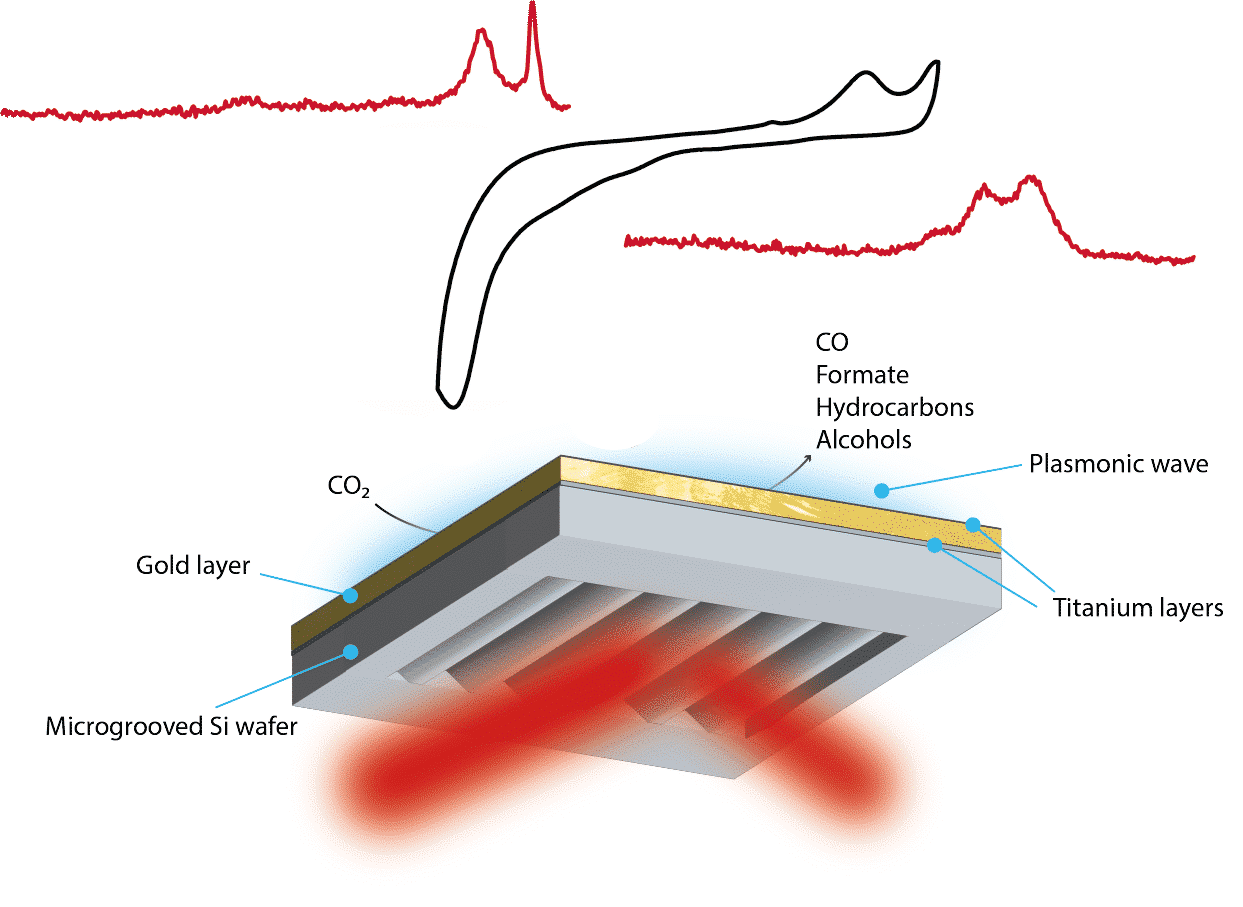
The energy transition presents many challenges. Examples are the search for alternative energy carriers that do have the desirable properties of hydrocarbon-based fuels such as high energy density, but without the accompanying carbon dioxide emissions. Another example is the large-scale storage of renewable electricity, and finding alternative, lower-emission processes for the production of our most in-demand chemicals, such as ammonia. Fundamental details of the involved catalytic reactions are often lacking, yet can be imperative to design the better catalysts and processes we need in order to bridge the societal challenges we currently face. The challenges that arise in trying to unravel details of catalysts under working conditions are generally exacerbated in electrocatalysis. Here, we will discuss the application of state-of-the-art spectroscopy, microscopy and spectromicroscopy techniques across the catalytic board for CO2 reduction; from homogeneous electrocatalytic, to heterogeneous electrocatalysis, and thermocatalysis. Via the application of inter alia in-situ high-resolution STEM, combined with quick-X-ray absorption spectroscopy with sub-second time resolution under reaction conditions, along with theoretical chemistry, we have exposed novel fundamental insights of (electro-)catalysts at work, such as reaction mechanisms, and structure-activity relationships. This contributes to our ability to produce “ideal” catalysts by improving the understanding of the catalytic sites and reaction pathways responsible for higher activity and even C-C coupling to higher value-added products. This toolbox provides new insights for e.g. CO2 activation towards value-added chemicals thereby reducing the deleterious effects of this environmentally harmful molecule.