
NON-CANONICAL BINDING TO MICROTUBULES REGULATES THE IN VIVO AND IN VITRO FUNCTIONS OF A BI-DIRECTIONAL KINESIN-5 MOTOR
2Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, USA
3Department of Life Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel
4The National Institute for Biotechnology in the Negev, Ben-Gurion University of the Negev, Beer Sheva, Israel
The plus-end directed motility of kinesin-5 motors is essential for their functions in mitotic spindle dynamics. Surprisingly, several fungal kinesin-5 motors were reported to move in the two directions on the microtubules (MTs) under different experimental conditions. Although such bi-directional motility was proposed to be important for mitotic functions, the underlying molecular mechanism remains unknown. The bi-directional kinesin-5 motors contain two unique structural elements: the N-terminal non-motor extensions, that are conserved in bi-directional kinesin-5 motors, and unstructured loop8 (L8) region in the motor domain, that is conserved among the Saccharomycetaceae family of yeast, both not found in exclusively plus-end directed kinesin-5 and kinesin-1 motors.
To understand the role of these structural elements in regulating the function of bi-directional kinesin, we used single molecule motility assays, multi motor gliding assays, and live cell imaging, to examine the motile properties of different mutants of bi-directional kinesin-5 Cin8, carrying deletion in these regions. In addition, Cryo-electron microscopy (cryo-EM) was used to examine the structure of Cin8 motor domain, containing part of its non-motor N-terminal region.
We report that the large insert in L8 of Cin8 and the non-motor N-terminal region increase the MT affinity of Cin8 both in vivo and in vitro, and are required for Cin8 intracellular functions. In cells, Cin8 variants carrying deletion in L8 and the N-terminal region exhibited defects in localization to the mitotic spindle. Single molecule motility of these variants exhibited minus-end directed bias, consistent with their reduced affinity to MTs. Moreover, modeling and molecular dynamic simulation of the weak electron density obtained for the non-motor N-terminal region revealed that this region likely forms an α-helix and interacts with the β-tubulin subunit, likely at its C-terminus. Thus, the L8 and the N-terminal region create non-canonical binding sites to MTs, in addition to the conserved MT binding site of kinesin motors. We suggest that these regions facilitate flexible anchoring of Cin8 to the MTs required for bi-directional motility of single Cin8 molecules and are essential for intracellular functions. These additional non-canonical sites for MT binding demonstrated here for Cin8 is likely to be a common feature of bi-directional motors required for stepping in two directions.
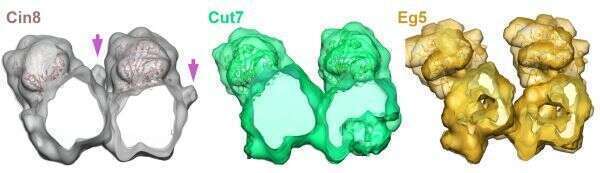
Electron density of the non-motor N-terminal region of Cin8. Cryo-EM electron density for Cin8 bound to MTs obtained in this work (light grey), compared to the cryo-EM density maps of Cut7 (green) and Eg5 (yellow), all bound to MTs. Cross section view, from minus- to plus-end of the MTs is presented. The most pronounced difference between the three structures is a volume of weak intensity in the Cin8 cryo-EM density map (arrow), which is absent from the density maps of Cut7 and Eg5.