Chemical Information Exchange Between Species:
Living Together by the Grace of Good Chemistry
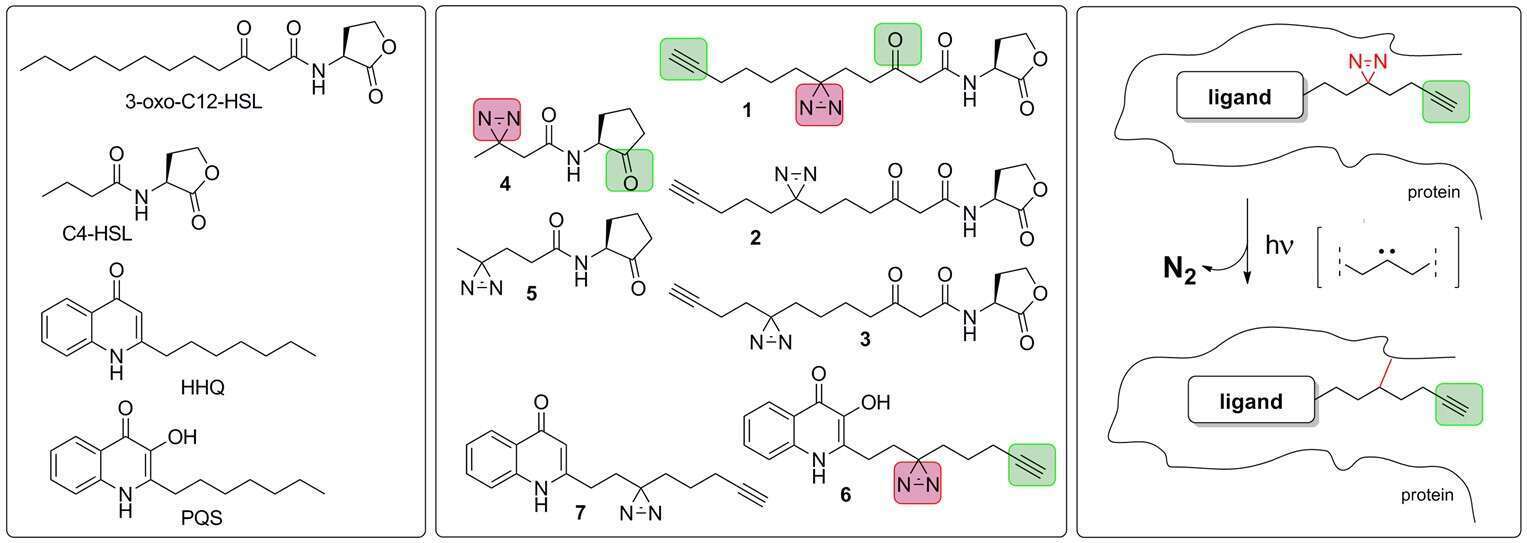
Life on earth is heavily based on chemical communication between cells. Quorum sensing enables unicellular organisms to coordinate their behavior and function in such a way that they can adapt to changing environments and compete, as well as coexist, with multicellular organisms. Prime examples of this phenomenon are displayed by the opportunistic pathogens Pseudomonas aeruginosa and Agrobacterium tumefaciens, which cause disease in humans and plants, respectively – but most often don’t. Quorum sensing in these pathogens is mediated by small amphiphilic signaling molecules such as 3-oxo-C12-HSL and 3-oxo-C8-HSL, leading to biofilm formation and secretion of virulence factors. The Meijler group is targeting QS in various pathogens with several chemical tools, such as a set of electrophilic and photoactivatable ‘tag-free’ probes that are designed to bind QS receptors covalently. These probes are used as molecular tools to obtain new insights into the mechanisms of activation, deactivation and recognition of bacterial quorum sensing – through the use of bioorthogonal chemistry. Diverse eukaryotes have been found to react strongly to the presence of these compounds, and the recognition of QSMs is mediated by mostly unknown receptors. Recently we identified and validated the role of a human receptor for HSLs, called the Major Vault Protein (MVP), as an important immunomodulator, and we expanded our toolbox of activity-based signaling probes in order to unravel interspecies communication in challenging niches such as the human gut.