
Nosological, Therapeutic and Prognostic Implications of Genomics in Juvenile Refractory Dermatomyositis: A Case Report
BACKGROUND
Juvenile dermatomyositis (JDM) is a multisystemic and autoimmune inflammatory pathology of variable and poorly explained aetiology, categorised as an inflammatory myopathy associated with defined skin lesions. Genetic susceptibility plays an important role in its development and the association of the disease has been determined with HLA alleles (B8, DRB1*0301, DQA1*0501 and DQA1*0301) and with polymorphisms in the TNF-α gene (1). Diagnosis has classically been based on the clinic supported by diagnostic and laboratory aids (2). However, genomic studies are becoming increasingly relevant, better characterising the role of rare and non-coding genetic variants in the various myopathies, thus leading to individualised management and clinical presentations of the disease (3).
We present a clinical case of juvenile dermatomyositis refractory to treatment, secondary to common variable immunodeficiency as a result of a variant in the TNFRSF13B gene to illustrate the nosological, therapeutic and prognostic implications of genomics in this disease.
CASE REPORT
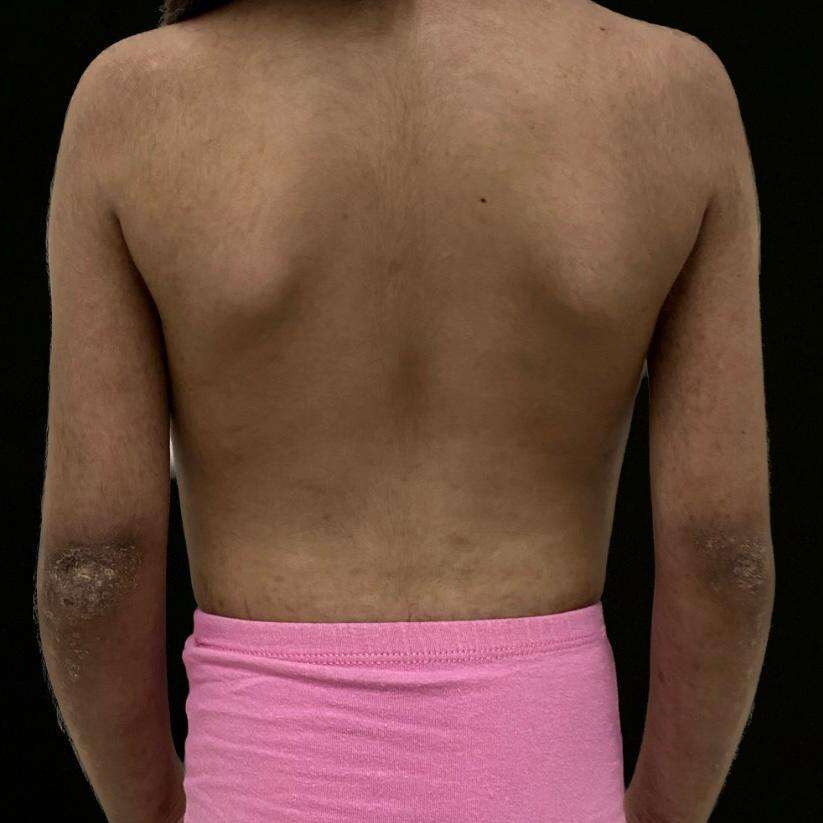
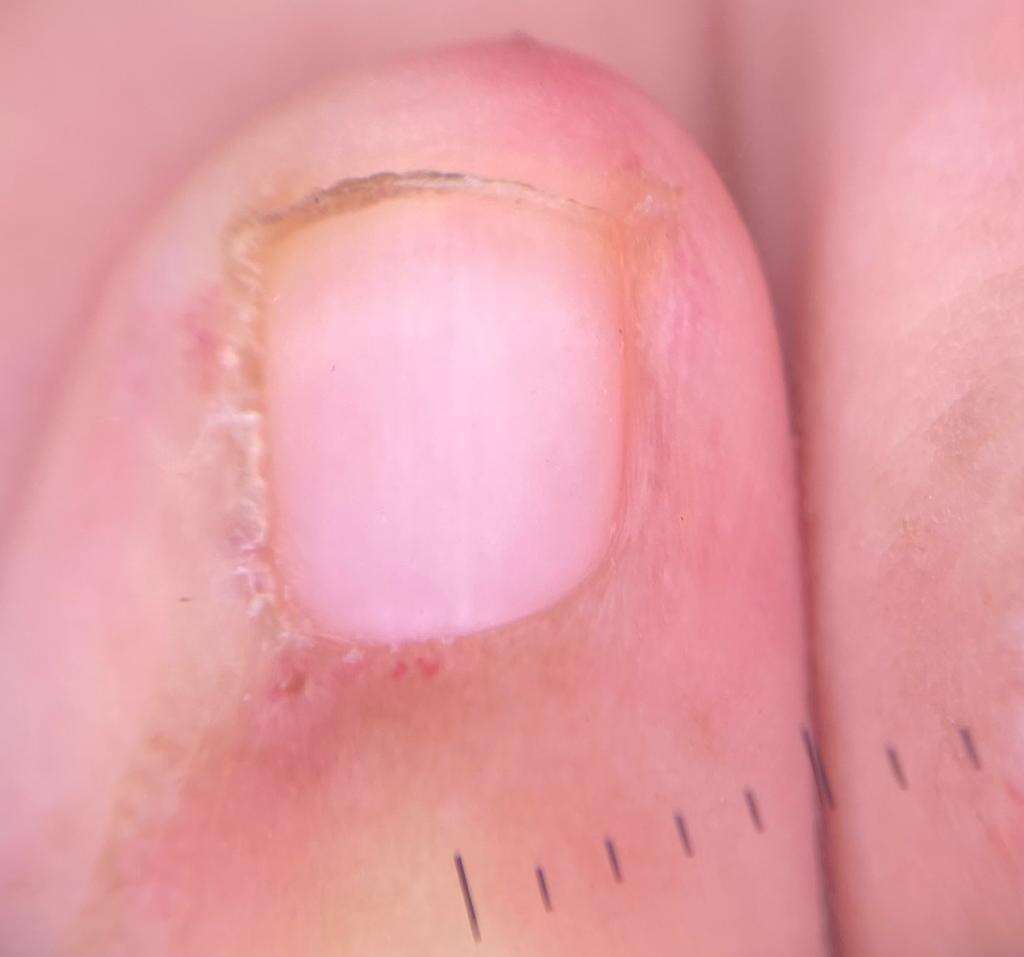
The 7-year-old female patient was the product of a first-term pregnancy of non-consanguinous parents, her mother had no relevant pathological history and her father was a controlled diabetic, both without a history of immunological or genetic diseases. The patient was diagnosed with juvenile dermatomyositis in June 2019 and had a double left renal collecting system with recurrent urinary tract infections. Her joints were managed with rheumatology, pediatric urology and physiatry, and initially, she was medically managed with prednisolone 7.5 mg/day, methotrexate 10 mg/week, chloroquine 125 mg/3 times a week, and folic acid 1 mg/4 times a week and had complementary management with physical and occupational therapy. After 6 months of follow-up, human immunoglobulin G therapy at 15gr/month was added to her treatment and due to persistent transaminitis despite established medical management, the patient was genetically evaluated to rule out immunodeficiency and expand studies to verify or rule out the genetic aetiology of the condition and establish a targeted treatment and define prognosis (see attached images, reproduced with the permission of the patient’s mother).
RESULTS
The molecular sequencing study using NGS + CNV (copy number variants) for genes related to immunodeficiencies (228 genes) was recommended after pre-test counselling. A probably pathogenic heterozygous variant was found in the TNFRSF13B gene, consisting of the substitution of thymine for cytosine at position 310 in exon 3 (c.310TC). which, at the protein level, generates the missense change from cysteine to arginine in codon 104 (p.Cys104Arg), classified as probably pathogenic (ClinVar ID: 645207). The Human Gene Mutation database (HGMD) reported this as associated with common variable immunodeficiency (CVID) (CM052924), with autosomal dominant and recessive inheritance, therefore, there is a 50% probability that each child will inherit this variant. Post-test counselling was performed and a dermatology assessment revealed disease activity on the skin of active Gottron sign, generalised xerosis, some erythematous scaly plaques on the extremities and alterations in capillaroscopy. The methotrexate dose was increased to 15 mg/week with continued management with human immunoglobulin G at 15 g/month, and skin care measures such as moisturising and photoprotection were reinforced. The patient continues in interdisciplinary clinical follow-up with slight disease improvement.
DISCUSSION/CONCLUSION
TNFRSF13B was first described in 1997 and has an important role in plasma cell differentiation, isotype switching from IgM to other immunoglobulins, interferes with B cell response and survival, controls autoreactive antibody production, and balances B cell tolerance by regulating apoptosis (4). Biallelic and/or monoallelic variants of TNFRSF13B are associated with various pathologies such as common variable immunodeficiency (CVID) (5), selective IgA deficiency, lymphoproliferation disorders and some autoimmune disorders such as systemic lupus erythematosus (SLE) and dermatomyositis (6).
Regarding the CVID herein, it is included in the spectrum of primary immunodeficiencies with varied clinical manifestations and infectious and non-infectious complications. However, the association of CVID with rheumatic diseases and especially with JDM, due to the rarity of these manifestations, is still unclear (6) but it has been proposed in some cohorts that patients with rheumatic diseases and CVID have slightly higher basal IgG levels, a family history of autoimmunity, and increased autoantibody formation. The comorbidity of CVID and rheumatological disease has a poor prognosis due to the poor response to conventional therapies and immunoglobulin replacement therapies, requiring additional immunomodulatory treatments to manage these pathologies (7) as in the case presented.
It is at this key point where genomics plays a fundamental role, since although in CVID the first genetic studies identified autosomal recessive genes, autosomal dominant genes with variable penetrance have been described increasingly frequently, in up to 30% of cases. Sequencing studies for specific genes or whole exome sequencing (7) can provide opportunities for genetic counselling and individualised therapeutic options, thus improving their quality of life and disease prognosis.
REFERENCES
1. Plácido Paias, Raquel, Raúl Veroz González, and Manuel Portillo Márquez. "Dermatomiositis juvenil: a propósito de un caso." Pediatr. aten. prim (2022).
2. DeWane ME, Waldman R, Lu J. Dermatomyositis: Clinical features and pathogenesis. J Am Acad Dermatol. 1 de febrero de 2020;82(2):267-81
3. Lamb JA. The Genetics of Autoimmune Myositis. Front Immunol. 26 de mayo de 2022;13:886290.
4. Firtina S, Kutlu A, Yozlu M, Cepeci BN, Isikgil B, Ar MC, et al. Integrative Analysis of the TNFRSF13B Variants in Common Variable Immune Deficiency [Internet]. In Review; 2022 Sep.
5. Pérez MLP, Malda HIA, Martín OGP. Inmunodeficiencia Común Variable. Reporte de un caso. Rev Cuba Genética Comunitaria [Internet]. 28 de febrero de 2019.
6. Agarwal S, Cunningham-Rundles C. Autoimmunity in common variable immunodeficiency. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. noviembre de 2019;123(5):454-60.
7. Picard C, Bobby Gaspar H, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol. enero de 2018
FIGURES
.jpeg)
.jpeg)
.jpeg)
.png)