The acetylation of major plant hemicellulose, xylan, protects xylosidic linkages against the action of the corresponding glycoside hydrolases of plant invading microorganisms. Alkaline pretreatment used frequently to overcome the plant cell wall recalcitrance, also destroys the ester linkages, making glycosidic linkages accessible to enzymatic hydrolysis. Unable to apply alkaline pretreatment, microorganisms produce esterases hydrolyzing the ester linkages. These carbohydrate esterases (CEs), called acetylxylan esterases [1], face the task to release acetic acid from five unequivalent structural arrangements: from 2-O- and 3-O-monoacetylated Xylp residues, 2,3-di-O-acetylated Xylp residues and also from 3-O-acetylated Xylp residue substituted with MeGlcA at position 2 (Fig. 1) [2-4].
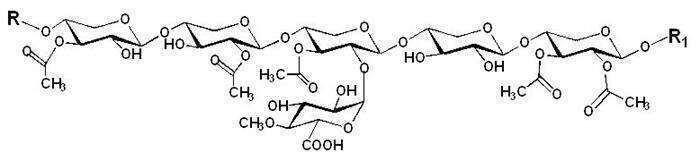
Fig. 1. Five unequivalent acetyl groups in hardwood acetylglucuronoxylan that can be distinguished by 1H-NMR spectroscopy.
3D-structures of most of the AcXEs families are available (CAZy), but we do not know enough about their mode of action and real function. An 1H-NMR spectroscopy approach has been introduced to follow the positional specificity of AcXEs on native partially acetylated xylan. It is based on monitoring the signal intensity of the acetyl groups [5]. All enzymes assigned for AcXEs on the basis of their ability to precipitate acetylglucuronoxylan from solutions [1], are surprisingly capable to deacetylate the positions 2 and 3 in monoacetylated Xylp residues. This positional non-specificity has been observed with both serine-type and aspartate-type esterases. Members of some CE families also attack 2,3-di-O-acetylated Xylp residues, however, none of so far tested AcXEs was found capable of deacetylating Xylp residues α-1,2-substituted with MeGlcA. AcXE with such specificity has to be discovered.
[1] Biely P. Biotechnol. Adv. 30, 1575-1588, 2012.
[2] Teleman A. et al., Carbohydr. Res. 329, 807-815, 2000.
[3] Evtuguin D.V. et al. Carbohydr. Res. 338, 597-604, 2003
[4] Naran R. et al. Cellulose 16, 661-675, 2009.
[5] Uhliariková I. et al. Biochim. Biophys. Acta, in press.

