Polyene macrolide antibiotics (PMA) are a numerous group of natural compounds with activity against yeasts and yeast-like and filamentous fungi, both saprophytic and pathogenic species. However, PMA used in medical practice do not completely satisfy clinical requirements because of low efficacy and unsuitability for the treatment of various forms of deep mycoses, high toxicity, and lability on storage. In view of this, a search of new derivatives of PMA with improved medical and biological properties continues.
We have demonstrated that reactions of heptaene macrolide antibiotic mycoheptin1with aromatic aldehydes and hypophosphoric acid lead to the formation of its hydrophosphoryl derivatives 2-10. The reaction being studied may be considered to be a variant of the Kabachnik-Fields reaction. In the first stage of the process, the primary amino group of the mycosamine (3-amino-3,6-dideoxy-D-mannose) combines with the carbonyl group of the aromatic aldehyde, with the formation of an azomethine intermediate. In the second stage hypophosphoric acid reacts with the C=N bond of the azomethine intermediate, with the formation of hydrophosphoryl derivatives of mycoheptin 2-10.
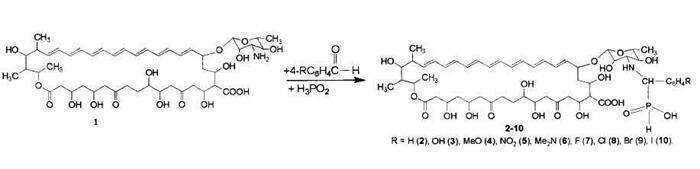
Biological studies showed that hydrophosphoryl derivatives of mycoheptin 2-10were less toxic than initial antibiotic and have expressed antifungal activity against 11 test-cultures of pathogenic fungi. Additional biological tests have also indicated high antiviral activity in relation to the DNA-containing vaccinia virus and two RNA-containing viruses: the oncogenic Rous sarcoma virus and influenza viruses type A and type B.

