Within the last years, metabolic oligosaccharide engineering (MOE) has been established as an important tool to monitor carbohydrates in vivo and in vitro.[1] Different ligation methods can be used to detect the unnatural sugars that have been metabolically incorporated. In addition to the well-established azide-alkyne cycloaddition, our lab also uses Diels-Alder reactions with inverse electron demand (DARinv) between an alkene and a tetrazine as ligation reaction (Figure 1a). One of the advantages of the DARinv is that it does not require catalysis by toxic metals and that the release of nitrogen during the reaction makes it irreversible. Moreover, the DARinv can be carried out in the presence of azides, and it is, thus, possible to label two different metabolically incorporated sugars in the same experiment.[2]
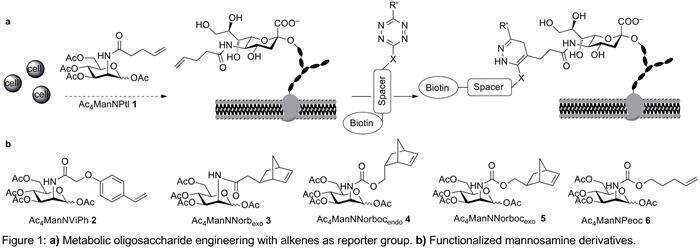
Here, we show the synthesis and evaluation of several new mannosamine derivatives (Figure 1b) containing dienophiles that may improve the efficiency of the DARinv. Monitoring the reaction kinetics we could confirm that electron-rich (2) or strained (3-5) alkenes react faster in the DARinv than terminal alkenes. Furthermore, we could show that in addition to the mannosamine derivatives with an acyl chain connected to the 2-amino group, the biosynthetic machinery also accepts derivatives with a carbamate group (6). A carbamate group has the advantage that it facilitates the synthetic access to functionalized mannosamines derived from alcohols, such as pentenol.
[1] a) J. A. Prescher, C. R. Bertozzi, Cell 2006, 126, 851-854; b) D. H. Dube, C. R. Bertozzi, Curr. Opin. Chem. Biol. 2003, 7, 616-625; c) O. T. Keppler, R. Horstkorte, M. Pawlita, C. Schmidt, W. Reutter, Glycobiology 2001, 11, 11R-18R.
[2] A. Niederwieser, A.-K. Späte, L. D. Nguyen, C. Jüngst, W. Reutter, V. Wittmann, Angew. Chem., Int. Ed. 2013, DOI: 10.1002/anie.201208991

